Scroll to:
Atherosclerosis pathogenesis from the perspective of microvascular dysfunction
https://doi.org/10.15829/1728-8800-2021-3076
Abstract
The article discusses different points of view on atherosclerosis development. The facts confirming the lipid hypothesis are presented. Attention is drawn to the possible participation of vasa vasorum in the development of atherosclerosis.
For citations:
Aronov D.M., Bubnova M.G., Drapkina O.M. Atherosclerosis pathogenesis from the perspective of microvascular dysfunction. Cardiovascular Therapy and Prevention. 2021;20(7):3076. https://doi.org/10.15829/1728-8800-2021-3076
It is well known that important discoveries were accompanied by hard work to be recognized by their contemporaries. Anichkov N. N. did not escape this fate, having created the cholesterol theory of atherosclerosis, which is effective in the treatment and prevention of atherosclerosis. In 1913, he published an article in the pathological journal “Zentral Allg Pathol Pathol Anat” on the central role of cholesterol in experimental atherogenesis [1].
For a long time, the work of Anichkov N. N. remained unknown. Fame came to him in the 30s of XX century, when he presented at the conference of International Society of Geographical Pathology data on the prevalence of atherosclerotic lesions in different countries, depending on social and professional characteristics. This was the first serious work in the world that laid the foundations of medical epidemiology, which developed as one of the merits of Anichkov N. N. But the most important merit of Anichkov N. N. is considered to be his creation of the cholesterol concept of atherosclerosis [2][3].
Currently, prevention and treatment of atherosclerosis are carried out precisely according to the methodology of Anichkov N. N. The leading role of cholesterol in atherosclerosis is the need to control its level and reduce the elevated level to the established target values. Unfortunately, his discovery was pursued by failures with Back in 1992, Academician A. N. Klimov reproached a certain Davis N., who spoke about the concept of atherosclerosis and personally opposed Anichkov N.N. [4]. In a review published in 1990 in the International Journal of Cardiology, Davies H expressed an derogatory attitude towards the cholesterol concept of atherogenesis and its discoverer [5]: “It is of some interest to note, finally, that the feeding of cholesterol to rabbits, which ushered in the lipid era, began in Russia at about the same time as the Bolshevik Revolution. Both, in their different ways, have inf luenced the course of mankind in this century, if only in the sense of denying other and better things. It would be of even more interest if the end of the century should see the simultaneous decline of both systems and the theses which they have spawned, for they have proved less than satisfactory”. Note that it is hardly appropriate to compare such a social phenomenon as the October Revolution in Russia and a purely scientific discovery — the creation of a cholesterol model of atherosclerosis in rabbits by Anichkov N.N. and Khalatov S.S. in 1913 [4]. In addition, Klimov A.N. presented headings of articles criticizing Anichkov N. N. [5] (information about the authors according to Klimov A.N.):
- “Cholesterol controversy — where do we go from here?” (Heyden S, Williams S, 1982);
- “Serum-cholesterol the knave of hearts and the joker” (Oliver MF, 1981);
- “The Cholesterol Saga: Whither Health Promotion?” (Becker MH, 1987);
- “National Cholesterol Education Program: does the emperor have any clothes?” (Palumbo PJ, 1988);
- “The cholesterol myth” (Moore TJ, 1989), etc.
Until now, different views on atherosclerosis pathogenesis have been expressed. The authors of the recently published book “Atherosclerosis Pathogenesis and Microvascular Dysfunction “ (2019) Haverich A and Boyle EC believe that atherosclerosis primarily originates in microvascular tissues (vasa vasorum, lymphatic vessels) and only later the process is transferred to the intima of coronary and other arteries [6]. This path of atherogenesis for the first time draws the attention to arterial wall, not only in the intima, but in the medial layer and adventitia. The authors support their concept of atherosclerosis development with a huge list of literature (886 articles), including works of the 19th, 20th and 21st centuries.
The main idea of these authors is that atherosclerosis is not a consequence of hypercholesterolemia. They continue further: “…and, although most studies are focused on the endothelium of main vessel, we substantiate the hypothesis that it is the vasa vasorum endothelium that is more susceptible to dysfunction at all disease stages” [6]. In their opinion, the primary cause of atherosclerosis is pathology of vasa vasorum, which are defined literally as vessels of vessels. Vasa vasorum are microvessels that supply the walls of moderate and large arteries, as well as veins with nutrients and oxygen, and also participate in the removal of natural metabolic products and elements involved in pathological processes in the vascular wall. Vasa vasorum do not form plexuses, but rather play the role of functional end arteries.
A more intensive study of vasa vasorum is certainly useful, but this does not mean that its dysfunction is of decisive importance in atherosclerosis pathogenesis, where cholesterol is the main factor. According to Haverich A and Boyle EC, “ atherosclerosis develops from adventitia to intima”, but not vice versa, as it is still believed. Considering the pathogenesis of atherosclerosis in a new way, the authors almost completely revise the mechanisms of its development [6]. The authors rely on some of their own results, which are of no particular importance in overthrowing the cholesterol theory of atherosclerosis. Another part of their own results is supported by extensive new material on cardiac microvessels, little known to clinicians.
Figure 1 shows the vasa vasorum development according to the concept of Haverich A and Boyle EC [6]. Vasa vasorum originate from all large arteries up to the bifurcation segments of the epicardial vessels. There are also venous vasa vasorum, which divert blood from the arterial system to venous system. The vasa vasorum system develops gradually from birth to adulthood and is similar to annual growth rings in a tree (Figure 1). Over time, the number of layers of vasa vasorum intima cells increase as follows: from 1-2 layers in the first year of life to 10-15 layers by the age of 15 and further to 25- 30 layers in adulthood. This process is associated with intima state, which diffusely thickens over the years (this is called diffuse intimal thickening). This phenomenon was discovered by Kapitolina Volkova, a student of Anichkov N.N. It is known that adaptive intimal thickening does not depend on hypercholesterolemia. Atherosclerotic plaques develop from an already thickened intima. It is believed that if the critical value of thickening is exceeded, then hypoxia in the outer part of tunica media and adventitia will lead to the triggering of angiogenesis and the vasa vasorum formation [6].
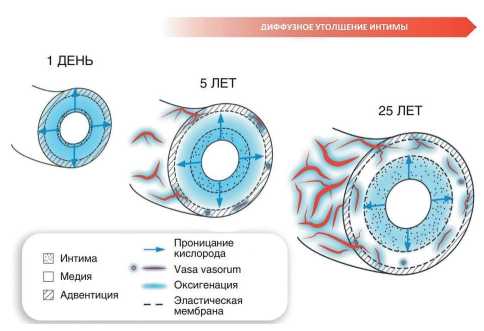
Figure 1. Age-related development of vasa vasorum facilitates nutrition and oxygenation of vascular wall layers.
Vasa vasorum in young people first appear only in the adventitia and with age, with intimal thickening, vasa vasorum penetrate into the outer two-thirds of the media, the adventitia and the outer layer of the media. At the same time, the blood supplies nutrients to vessel itself, including the inner layer of artery (only the local artery part is supplied with blood). The treated area of artery is autonomous and is in no way connected with areas of perfusion of other parts and other vessels, except for a limited part under the intima. Vasa vasorum can be subjected to short-term compression by arterial wall in the presence of edema. They regulate their tone and vascular perfusion, since they are enriched with their own smooth muscle cells and connective tissue [7].
Three-layer vessel wall structure is in all arteries with some differences. The outer arterial layer (adventitia, or tunica externa) consists of collagen-rich connective tissue with elastic fibers containing fibroblasts, nerve, perivascular, Marchand’s cells, macrophages, T-lymphocytes, mast and dendritic cells. The second layer (tunica media) is separated from the adventitia by an elastic membrane. The media consists of circularly arranged plates of smooth muscle cells, connective tissue and elastic fibers. The inner layer (intima, or tunica interna) consists one layer of endothelial cells and a connective elastic layer — subendothelium. In recent years, stem and other type cells (macrophages, follicular and dendritic cells) have been found in all arterial wall layers.
The lymphatic system is represented by a network of initial lymphatic capillaries that extended through the postcapillary lymph vessels to collecting ones. They consist solely of endothelium, which is attached by collagen fibers to the basement membrane. Fluid enters these “blind” capillaries through absorption, which promotes contacts between permeable endothelial cells [6]. In addition, the pressure gradient affects the fluid movement from the interstitial space into the lymphatic capillaries. Lymphatic vessels are involved in the absorption and metabolism of fats, as well as in immune metabolism. Excess cholesterol is removed from the cells within high density lipoproteins and transported by lymph into the blood and back to the liver, from where cholesterol is excreted from the body. This fact indicates the positive role of lymph in the prevention of atherosclerosis. Lymphatic vessels have a separate blood supply with vasa vasorum [6].
One can agree with the opinion of Haverich A and Boyle EC that microvessels in the blood and lymphatic vessel walls do not receive due attention of researchers and their pathology is often ignored. Indeed, for many years scientists have rarely paid attention to the role of vasa vasorum in atherogenesis.
Some of the issues highlighted by Haverich A and Boyle EC require 3D artery visualization using optical coherence tomography and magnetic resonance imaging. When studying images obtained from patients with atherosclerosis and calcified arteries, their significant heterogeneity is revealed. Thus, it is obvious that microvessels do take a certain part in the blood supply to cardiac arteries, but by no means its main part [6]. Technological progress in computed tomography has made it possible to verify atherosclerotic lesions. Detailed comparisons between the left and right aortoiliofemoral axis obtained in patients with calcified arteries in many cases show a symmetrical bilateral calcification. At the same time, there is a pronounced asymmetry in the calcified vessels of lower half body. In these cases, the main segments of arterial circumference do not actually have atherosclerotic changes.
Based on the images obtained using computed tomography, another important observation can be made: plaques in a certain arterial segment are distributed unevenly. Rather, a point or fragmentary distribution of calcifications is seen. In addition, calcification is not found in the visceral arteries, as well as in the depth of femoral artery further than 1-2 cm after its exit from the common femoral artery. Thus, on the one hand, there is symmetry between the left and right iliac and femoral arteries, on the other hand, there is minimal atherosclerosis symmetry in certain segments of lower limb arteries. It was determined that in larger arteries the disease is much more pronounced than in small ones.
It is well known that the mammary artery, with very rare exceptions, is not affected by atherosclerosis. The same applies to the intramural coronary arteries, which are always free from atherosclerotic involvement, even in patients with very extensive calcified and noncalcified plaques in coronary arteries. These data led the authors Haverich A and Boyle EC to believe that such differences reflect fundamental features of atherosclerosis pathogenesis, which are contrary to the dogma that atherosclerosis is a systemic disease [6].
The famous immunologist and Nobel Prize laureate for 1908 Mechnikov I.I. suggested that excess protein in the human diet is potentially toxic and accelerates the aging process. Inspired by this hypothesis, a young experimental pathologist Ignatovsky A.I., who worked at the Military Medical Academy in St. Petersburg, conducted experiments on rabbits, which were fed a lot of meat, eggs and milk. He found that in rabbits fed a protein-rich diet, in addition to changes in the liver, spleen, and kidneys, there were pronounced changes in the arteries, reminiscent of atherosclerosis in humans. Later, he gradually narrowed down the range of nutritional factors and showed that vascular changes were caused by cholesterol isolated from egg yolks. Following this work, his colleague, Anichkov N.N., tested the idea that these changes are related to cholesterol exposure and began experiments in which he fed purified cholesterol to rabbits. He found that cholesterol causes arterial changes that look similar to human atherosclerosis seen at autopsy. Therefore, Anichkov N. N. was convinced that food cholesterol was the cause of atherosclerosis. He slightly revised his theory in 1924, and the result was the “cholesterol hypothesis” or “lipid hypothesis” — the concept that high blood levels of cholesterol lead to the deposition of lipids on blood vessel walls [6].
“Atherosclerosis is not a generalized disease” — this thesis of N.N. Anichkov’s opponents is important for various reasons. A generalized disease is presented to them as a disease that proceeds standardly in all ways. If plaques appear in some vessels due to illness, they should be in all vessels. The non-generalization of atherosclerosis in the understanding of Haverich A and Boyle EC allows them to discredit coronary artery disease (CAD) as not identical to coronary atherosclerosis. In their opinion, CAD is not related to atherosclerosis. As a result of this approach, numerous major studies, especially in the last two or three decades, can be considered “worthless” [8][9].
As known, in practical medicine and scientific literature, the term “coronary artery disease” is used as a kind of analogue of coronary atherosclerosis. A number of studies discuss myocardial infarction (MI) in the absence of arterial stenosis, i.e. the so-called myocardial infarction with nonobstructive coronary arteries (MINOCA). MINOCA is a syndrome characterized by clinical signs of MI with normal coronary angiography; arterial stenosis up to 50% is allowed [10-12]. Its diagnosis is quite difficult, and to a certain extent impossible. According to Niccoli G and Camici PG (2020), the prevalence of such MI is ~10% (more precisely, within 4,6-11,1%), and this is a fairly small part of all cases of acute MI [10]. At the same time, 90% of acute MI and acute coronary syndrome (ACS) are quite accessible for accurate diagnosis and proper treatment. It’s easy to accept that diagnosis of CAD does not always match its name, but to indiscriminately deny its merits would be a much more serious mistake than to do without it.
The mechanisms underlying MINOCA are diverse. A recently proposed classification distinguishes between epicardial (vulnerable plaques undetectable on angiography, epicardial spasm or dissection coronary arteries) and microvascular causes. The latter, in turn, can be divided into internal (microvascular spasm, Takotsubo syndrome and coronary embolization) and external (myocarditis). Vulnerable plaques and coronary artery dissection, rupture or erosion of plaques fall under type I MI. Dysfunctional microcirculation causes myocardial necrosis followed by luminal compression due to myocardial edema. Takotsubo cardiomyopathy often presents as ACS with ST segment abnormalities and is usually accompanied by elevated levels of myocardial necrosis markers. The clinical performance in some cases may be more severe, with acute heart failure up to shock.
The prognosis of MINOCA is variable and depends on the underlying cause with high-risk clinical subgroups. The correct diagnostic procedure includes first line tests (clinical/anamnestic, electrocardiography, myocardial necrosis markers, echocardiography, coronary angiography, ventriculography) and second line tests (intracoronary angiography, coronary vasomotor test, cardiac magnetic resonance imaging). MINOCA mortality, according to different authors, is significantly lower than in conventional types of MI: 1,1-5,8% vs 2,1- 14% [10].
N. N. Anichkov’s experiments on feeding of cholesterol to rabbits were the first in which dietary fats were associated with the disease process. This idea was later developed in the 1950s, but it was again seriously revised [8, 9, 13]. In these articles, the headlines are already striking in their rudeness.
Speaking about atherosclerosis, a number of authors are trying to debunk some, from their point of view, incorrect ideas. First, not only people suffer from atherosclerosis, but also many animal species: herbivores, omnivores and predators. Second, it regards the significance of such risk factors (RFs) as obesity, low physical activity, and a high content of saturated fats in the diet in the atherosclerosis development.
There is nothing special about the fact that some animals also have atherosclerosis. On the contrary, this fact indicates a higher prevalence of atherosclerosis in our world. In a number of animals, atherosclerosis develops with hypercholesterolemia, which once again proves the close relationship between atherosclerosis and elevated blood levels of cholesterol. Opponents argue that atherosclerosis is actually an ancient disease, since severe atherosclerosis has been found in many mummies. Thus, in the HORUS study (named after the ancient Egyptian god Horus), atherosclerosis signs were found in mummies of the Egyptians, Peruvians, Ancestral Puebloans mummies, and Aleuts; they all represent a wide variety of time periods, geographic regions, and types of nutrition [14]. Atherosclerosis was found in 37% of all mummies, the average age of which was 37 years.
The presence of atherosclerosis in ancient mummies in no way disputes the idea that atherosclerosis is a disease that is inextricably linked with lifestyle. Human life in all eras was accompanied by all sorts of hardships, hunger, tragic situations, various diseases and, of course, great physical stress. By the way, mummies who had atherosclerosis, it turns out, were among the nobility and during their lifetime were lovers of tasty and fatty food [14].
Against the background of identical systemic risks of atherosclerosis, certain anatomical regions of the arterial system with pronounced and dangerous atherosclerotic abnormalities are known, while other areas are never involved. This raises some questions, which result in a critical look at the modern pathogenesis of atherosclerosis, namely: what local factors protect certain parts of arteries from atherosclerosis against the background of systemic influence of RFs? It is really difficult to answer this question. First of all, because there is a number of questions that have some kind of logical justification.
Over the years, more and more studies have appeared that showed a link between the cholesterol amount in diet and atherosclerosis, although a number of cardiologists and nutritionists continued to be skeptical, since, from their point of view, there was no evidence [15].
W Stehbens is one of the most ardent opponents of cholesterol theory, who argues that the hypothesis of a link between cholesterol and heart disease is based on false premises, such as the incorrect use of the term “CAD” as an equivalent of coronary atherosclerosis, false data on mortality, a systematic error in recruiting patients by age, and zealous researchers [8][9]. The last words hint at a fake. Another opponent of Anichkov N. N. DuBroff R criticizes at the same time treatment with statins as falsely interpreted, since the drugs reduced mortality due to CAD, and not coronary atherosclerosis; they often did not affect all-cause mortality [16].
N.N. Anichkov’s opponents are surprised that most researchers believe that the reduction in cardiovascular mortality in countries such as the United States, where statins are widely used, is a consequence of their use. The authors believe that other RFs may have contributed, such as smoking cessation, which also declined. Switching to a Mediterranean diet, according to several studies, was as effective as taking lipid-lowering drugs [17][18].
A large study by Cho Y, et al. (2020) [19] provides evidence for the importance of statins in reducing mortality. The study followed up 81729 older Asians ≥75 years without evidence of CAD. To study the
effectiveness of statins among the elderly, the authors included 3670 patients who took statins from 2012 to 2014 in a ratio of 1:2 with the control group of people who did not take statins. In patients without statin therapy, there were 206 MIs, 1025 strokes, and 761 deaths. In patients taking statins, adverse cardiovascular events were observed less frequently as follows: 116 MIs, 637 strokes, 137 deaths. All differences in outcomes between the two comparison groups are highly significant. This latest result shows the effectiveness of statins in reducing not only acute MIs and stroke, but also all-cause mortality [19].
Often the Anichkov N.N. work was criticized due to extremely high cholesterol levels in rabbits (500-1000 mg/dl). Comparable results have never been obtained in non-herbivorous animal species, such as dogs or rats.
As already mentioned, the Anichkov N. N. works remained almost unknown for many years. The only publication in English prior to 1950 was a chapter in the first edition of Arteriosclerosis by Cowdry (1933). Only in 1950, N. N. Anichkov’s work on cholesterol and atherosclerosis received international recognition, which happened after the publication by Gofman J, Lindgren FT, entitled “The role of lipids and lipoproteins in atherosclerosis” [20].
In this paper, the authors specifically stipulate that Anichkov N. N. was the first to discover the relationship between cholesterol levels in the diet and vascular diseases, and report that, using his methods,
they confirmed and expanded conclusions. It is worth recalling that the group of Gofman J and Lindgren FT was the first to successfully use analytical ultracentrifugation to separate blood serum into low-density lipoprotein (LDL) and high density lipoprotein fractions [21]. Using this method, they showed a close correlation of LDL with atherosclerosis in both rabbits and humans [22]. This aroused great interest in research on atherosclerosis induced by cholesterol, and in the works of Anichkov N.N. 40 years after he made the first discoveries in this area.
Opponents of cholesterol hypothesis often negatively evaluate the studies of many well-known scientists and especially Keys A, who is one of the most famous for large studies. In 1953, he published data on the relationship between mortality from degenerative heart disease and the percentage of fat in diet for the population of six different countries (Japan, Italy, England and Wales, Australia, Canada and USA) [23]. In men aged 55-59 years, a close relationship was found between the percentage of fat in the daily calorie intake and mortality due to heart disease. In this regard, Keys A concluded that the diet, i.e. nutritional factor was the main contributor to blood lipid levels [24]. Keys A’s findings were immediately criticized by Yerushalmy J and Hilleboe HE [25].
Keys A was unfazed by this, and in 1958 he began the study “Coronary heart disease in seven countries”. This was the largest clinical study that prospectively examined the relationship between lifestyle, diet, and the prevalence of cardiovascular diseases (CVDs) for almost 30 years in middle-aged men from the United States, Japan, Greece, Yugoslavia, Italy, Finland, and Netherlands [26]. These countries were selected due to different lifestyles, dietary habits, prevalence of risk factors and chronic heart diseases, and mortality rates. Opponents of cholesterol hypothesis suggested that these countries could be “selected” on the basis of previously obtained results [25].
In the early 1980s, a large double-blind, randomized study was initiated in the United States to assess the effect of lowering plasma cholesterol levels through drug therapy, sponsored by the US National Institutes of Health. A significant reduction in cardiovascular endpoints has been demonstrated after lowering blood cholesterol levels with medications. All participants in the study had cholesterol-free diet. Therefore, the study did not directly assess the impact of diet-induced cholesterol reduction. Despite this, after the publication of the study results, the US National Institutes of Health organized a consensus conference, where a recommendation was made to limit the amount of cholesterol in the diet and follow a low-fat diet with a predominance of unsaturated fatty acids [27]. The idea that cholesterol causes atherosclerosis became mainstream in the 1990s. It was the time of cholesterol hypothesis triumph and outstanding success in its treatment with statins, other effective medications and non-drug methods.
The cholesterol hypothesis states that high blood LDL-C concentrations cause atherosclerosis, and if this is true, a decrease in LDL-C would be expected to reduce or at least stop atherosclerosis. Active cholesterollowering therapy for atherosclerosis has become standard medical practice after studies with statins showed a reduction in mortality from CAD [28]. It has been suggested that the initially recommended LDL-C target <100 mg/dL (2,6 mmol/L) should be reduced to <70 mg/dL (1,8 mmol/L) or <55 mg/dL (1,4 mmol/L) [29].
At the same time, data from numerous epidemiological studies were accumulating, which supported the hypothesis of the role of infection in CVD development. Antibody titers to specific pathogens correlated with the risk of atherosclerosis, CVD, and mortality from them [30][31]. However, contradictory data were also obtained, according to which there was no relationship between the serological response to infections and CVD.
One of the strongest associations between acute infection and cardiovascular events is that between MI and respiratory infections, especially inf luenza and community-acquired pneumonia. Acute MI is more likely to develop in winter, when the number of respiratory infections increases [32]. A meta-analysis of 16 epidemiological studies found a significant relationship between a recent respiratory infection and cardiac arrest [33]. The fact that influenza and pneumococcal vaccinations significantly reduces the CVD incidence is another indication that acute infection plays a role in heart damage [34].
Some of the most significant associations between chronic infection and CVD include H. pylori [35]. There is also strong evidence that periodontitis is associated with CVD [36]. Poor dental health is associated with an increase in MI incidence, while the risk of CAD increases with the severity of periodontitis [37]. A Danish study found that patients with periodontitis had twice the risk of CVD as patients with healthy gums [38]. Recently, a large Dutch study of 60174 participants concluded that periodontitis is an independent risk factor for atherosclerosis [39].
The role of plaque inflammation has been discussed for a long time, starting from the middle of the 19th century with Virchow R. ideas [40]. Macroscopically visible inflammation in the epicardial coronary arteries during surgical revascularization in patients with ACS was one of the initial stimuli for Haverich A and Boyle EC [6]. This local inflammation at the site of acute coronary occlusion was also noted by interventional cardiologists in Greece. They described a marked temperature increase in ACS at the site of occlusion compared to other arterial areas without atherosclerosis. We now know that inflammation itself plays an important role in all stages of atherosclerosis, including the onset, progression, and finally development of thrombosis [6].
C-reactive protein (CRP) is a component of acutephase response to inflammation and is used as its marker. In epidemiological studies, serum CRP level determined by a highly sensitive method, which gives the so-called high-sensitivity CRP (hsCRP), makes it possible to accurately predict the risk of adverse cardiovascular events [41][42]. However, studies in individuals with CRP gene variations that result in different plasma CRP levels clearly show that it is not involved in atherosclerosis pathogenesis.
The idea that inflammation underlies the atherosclerosis development is not unreasonable in light of other risk factors previously identified. Indeed, inflammation often accompanies many of the risk factors for atherosclerosis. For example, oxidized LDLs and crystalline cholesterol activate the NLRP3 inflammasome, which leads to caspase-1-mediated activation and secretion of pro-inflammatory cytokines of the interleukin (IL)-1 family [43].
Due to the pro-inflammatory nature of adipose tissue, obesity is associated with increased systemic inflammation [44]. Inflammation is believed to play a role in hypertension; infectious diseases, diabetes, chronic kidney disease, and smoking are also associated with elevated levels of systemic inflammation [45]. An association of clonal hematopoiesis with atherosclerosis risk has been reported. In mouse models of clonal hematopoiesis of indeterminate potential (CHIP), macrophages have increased expression of some chemokines and pro-inflammatory cytokines [46][47]. Therefore, inflammation is a phenomenon common to almost all identified RFs for atherosclerosis. Therefore, it has been suggested that it is a reasonable target for both preventive and curative interventions.
The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) investigated whether reducing inf lammation in patients with prior MI could reduce the risk of future cardiovascular events [48]. Canakinumab focuses at suppressing the activity of IL-1β, which is part of inflammasome (NLRP3) that interacts with cholesterol. This leads to increased inf lammation. Using a monoclonal antibody that neutralizes IL-1β (canakinumab), the researchers found no change in LDL-C levels, but noted a significant decrease in hsCRP and IL-6 levels. IL-6 is another inflammatory cytokine that is activated at the next signaling pathway stages. Patients treated with canakinumab had a ~15% reduction in the risk of MI, stroke, or cardiovascular death, and a ~30% reduction in prevalence of stenting or coronary artery bypass grafting [49]. Thus, this study provided the first direct evidence that inf lammation contributes to atherosclerosis development.
It is important to note that all-cause mortality in the canakinumab group increased significantly due to an increase in the incidence of fatal infections. Therefore, tactics aimed at combating inflammation should always take into account the presence of inflammation for proper immune protection and recovery, and not just its negative impact on atherosclerosis development.
Any high-quality epidemiologist can remind us that risk factors are not necessarily disease-causing factors. For example, draining swamps reduces the incidence of malaria. However, one cannot falsely conclude that swamps cause malaria: in fact, it is the malaria mosquitoes breeding in swamps that are responsible for infection spread [6].
We consider it appropriate to quote the following statement of Haverich A on this issue: “Based on my surgical experience, I have been critical of many hypotheses regarding the atherosclerosis pathogenesis, since two important paradigms seem to be wrong. First, atherosclerosis is not a systemic disease, since certain parts of the human arterial system are almost never affected by atherosclerosis. Secondly, the disease does not originate in the endothelium of involved vessel. A growing body of evidence and my own observations support the ‘outside-in’ theory in the initiation and progression of atherosclerosis, when vascular inflammation begins in the adventitia and spreads inward towards the intima” [6].
In fairness, we must agree that for many years science really did without mentioning the vasa vasorum role in the origin of atherosclerosis. Obviously, further research should give new results that are important for the completeness of atherosclerosis study, and open the door to readers about a little-known saga called “microvessels of the heart”.
There is an important question: where does atherosclerosis begin? In rare cases of infection aggression, apparently, there is the possibility of early onset of atherosclerosis in the adventitia and further spread to the intima. But this variant is not a classic variant of atherosclerosis and is rarely observed. As for the previously discussed problem of atherosclerosis generalization, “non-generalization” is better than “generalization”, because in the generalized variant, as shown by the Haverich A and Boyle EC [6], the disease course is more severe and, therefore, lead to greater mortality.
And finally, I would like to return to Anichkov N.N. and Khalatov S.S. in connection with the negative review of Davis N. A lot has been written about the studies of Anichkov N.N. and Khalatov S.S. Let us cite here only one statement by the famous American pathologist Dock W [50]: “The experiments carried out at the Military Medical Academy and leading to our modern knowledge of atherosclerosis are remarkable in many respects... The idea that a fatal disease can be due to an excess of food nutrients, is revolutionary. Therefore, the early work of Anichkov N.N. is comparable to the discovery by Harvey of blood circulation and Lavoisier of the respiratory exchange of oxygen and carbon dioxide. So, the scientific contribution of Anichkov N.N. and his associates was adequately assessed [50].
Relationships and Activities: none.
References
1. Anitschkow N, Chalatow S. Ueber experimentelle Cholesterinsteatose und ihre Bedeutung fuer die Entstehung einiger pathologischer Prozesse. Zentrbl Allg Pathol Pathol Anat. 1913;24:1-9.
2. Anichkov NN. Experimental arteriosclerosis in animals. In: Cowdry EV, editor, Arteriosclerosis: A survey of the problem. New York: MacMillan Publishing. 1933. p. 271-322.
3. Anitschow NN. Deuxieme conference international de pathologie geographyque. Oosthoek;1935.
4. Klimov AN. To the debate about cholesterol. Kardiologiia. 1992;2:3-8. (In Russ.)
5. Davies H. Atherogenesis and the coronary arteries of childhood. Int J Cardiol. 1990;28(3):283-91. doi:10.1016/0167-5273(90)90310-2.
6. Haverich A, Boyle E. Atherosclerosis Pathogenesis and Microvascular Dysfunction. Springer, 2019. 130 p. ISBN: 978-3-030-20244-6.
7. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease — six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33- 50. doi:10.7326/0003-4819-55-1-33.
8. Stehbens WE. Coronary heart disease, hypercholesterolemia, and atherosclerosis I. False premises. Exp Mol Pathol. 2001;70:103-19. doi:10.1006/exmp.2000.2340.
9. Stehbens WE. Coronary heart disease, hypercholesterolemia, and atherosclerosis II. Misrepresented Data. Exp Mol Pathol. 2001;70:120-39. doi:10.1006/exmp.2000.2339.
10. Niccoli G, Camici PG. Myocardial infarction with non-obstructive coronary arteries: what is the prognosis? Eur Heart J. 2020;22(Suppl E):E40-5. doi:10.1093/eurheartj/suaa057.
11. Lüscher TF. Revisiting angina pectoris with and without obstructive coronary artery disease. Eur Heart J. 2018; 39(23):2119-22. doi:10.1093/eurheartj/ehy335.
12. Kaski JC, Crea F, Gersh BJ, Camici PG. Reappraisal of Ischemic Heart Disease. Circulation. 2018;138(14):1463-80. doi:10.1161/CIRCULATIONAHA.118.031373.
13. McMichael J. Fats and atheroma: an inquest. Br Med. 1979;1:173-5. doi:10.1136/bmj.1.6157.173.
14. Allam AH, Thompson RC, Wann LS, Miyamoto MI et al. Atherosclerosis in ancient Egyptian mummies: the Horus study. JACC Cardiovasc Imaging. 2011;4(4):315-27. doi:10.1016/j.jcmg.2011.02.002.
15. Dayton S, Pearce ML. Diet high in unsaturated fat. A controlled clinical trial. Minn Med. 1969;52:1237-42. doi:10.1161/01.cir.40.1s2.ii-1.
16. DuBroff R, de Lorgeril M. Cholesterol confusion and statin controversy. World J Cardiol. 2015;7:404-9. doi:10.4330/wjc.v7.i7.404
17. Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62-9. doi:10.1001/jama.2011.907.
18. Åkesson A, Larsson SC, Discacciati A, Wolk A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol. 2014;64:1299-306. doi:10.1016/j.jacc.2014.06.1190.
19. Cho Y, Jeong Y, Seo DH, et al. Use of statin for the primary prevention of cardiovascular outcomes in elderly patients: A propensity-matched cohort study. Atherosclerosis. 2021;328:92- 9. doi:10.1016/j.atherosclerosis.2021.05.022.
20. Gofman JW, Lindgren F. The role of lipids and lipoproteins in atherosclerosis. Science. 1950;111:166-71. doi:10.1126/science.111.2877.166.
21. Gofman JW, Lindgren FT, Elliott H. Ultracentrifugal studies of lipoproteins of human serum. J Biol Chem. 1949;179:973-9.
22. Gofman JW, Jones HB, Lindgren FT, et al. Blood lipids and human atherosclerosis. Circulation. 1950;2:161-78. doi:10.1161/01.cir.2.2.161.
23. Keys A. Atherosclerosis: a problem in newer public health. J Mt Sinai Hosp N-Y. 1953;20:118-39.
24. Keys A, Anderson JT, Fidanza F, et al. Effects of diet on blood lipids in man. Clin Chem. 1955;1:34.
25. Yerushalmy J, Hilleboe HE. Fat in the diet and mortality from heart disease; a methodologic note. N Y State J Med. 1957;57:2343-54.
26. Keys A, Blackburn H, Menotti A, et al. Coronary heart disease in seven countries. Circulation. 1970; 41(Suppl 1):1-211.
27. Steinberg D. Lowering blood cholesterol to prevent heart disease. NIH Consensus Development Conference statement. Arterioscler Thromb Vasc Biol. 1985;5:404-12.
28. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383-9.
29. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140-205. doi:10.1016/j.atherosclerosis.2019.08.014.
30. Jha HC, Prasad J, Mittal A. High immunoglobulin A seropositivity for combined Chlamydia pneumoniae, Helicobacter pylori infection, and high-sensitivity C-reactive protein in coronary artery disease patients in India can serve as atherosclerotic marker. Heart Vessels. 2008;23:390-6. doi:10.1007/s00380-008-1062-9.
31. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, infammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363-71. doi:10.1093/aje/kwq177.
32. Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998;31:1226-33. doi:10.1016/s0735-1097(98)00098-9.
33. Barnes M, Heywood AE, Mahimbo A, et al. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101:1738-47. doi:10.1136/heartjnl-2015-307691.
34. Vlachopoulos CV, Terentes-Printzios DG, Aznaouridis KA, et al. Association between pneumococcal vaccination and cardiovascular outcomes: a systematic review and metaanalysis of cohort studies. Eur J Prev Cardiol. 2015;22:1185-99. doi:10.1177/2047487314549512.
35. Diomedi M, Pietroiusti A, Silvestrini M, et al. CagA-positive Helicobacter pylori strains may influence the natural history of atherosclerotic stroke. Neurology. 2004;63:800-4. doi:10.1212/01.wnl.0000138025.82419.80.
36. Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520-44. doi:10.1161/CIR.0b013e31825719f3.
37. de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. BMJ. 2010;340:c2451. doi:10.1136/bmj.c2451.
38. Hansen GM, Egeberg A, Holmstrup P, Hansen PR. Relation of periodontitis to risk of cardiovascular and all-cause mortality (from a Danish nationwide cohort study). Am J Cardiol. 2016;118:489-93. doi:10.1016/j.amjcard.2016.05.036.
39. Beukers NGFM, van der Heijden GJMG, van Wijk AJ, Loos BG. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60,174 participants in a large dental school in the Netherlands. J Epidemiol Community Health. 2017;71:37-42. doi:10.1136/jech-2015-206745.
40. Virchow R. Cellular pathology. 1860.
41. Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet (London, England). 2010;375:132-40. doi:10.1016/S0140-6736(09)61717-7.
42. Wennberg P, Wensley F, Di Angelantonio E, et al. Haemostatic and inflammatory markers are independently associated with myocardial infarction in men and women. Thromb Res. 2012;129:68-73. doi:10.1016/j.thromres.2011.05.015.
43. Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile infammation. Nat Immunol. 2013;14:812-20. doi:10.1038/ni.2639.
44. Després JP. Health consequences of visceral obesity. Ann Med. 2001;33:534-41. doi:10.3109/07853890108995963.
45. Altman R. Risk factors in coronary atherosclerosis atheroinflammation: the meeting point. Thromb J. 2003;1:1-11. doi:10.1186/1477-9560-1-4.
46. Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111-21. doi:10.1056/NEJMoa1701719.
47. Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 defciency accelerates atherosclerosis development in mice. Science. 2017;355:842-7. doi:10.1126/science.aag1381.
48. Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162:597-605. doi:10.1016/j.ahj.2011.06.012.
49. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119-31. doi:10.1056/NEJMoa1707914.
50. Dock W. Research in arteriosclerosis; the first fifty years. Ann Intern Med. 1958;49(3):699-705. doi:10.7326/0003-4819-49-3-699.
About the Authors
D. M. AronovRussian Federation
Moscow
M. G. Bubnova
Russian Federation
Moscow
O. M. Drapkina
Russian Federation
Moscow
Supplementary files
Review
For citations:
Aronov D.M., Bubnova M.G., Drapkina O.M. Atherosclerosis pathogenesis from the perspective of microvascular dysfunction. Cardiovascular Therapy and Prevention. 2021;20(7):3076. https://doi.org/10.15829/1728-8800-2021-3076

























































