Scroll to:
Role of biobanking in managing large-scale epidemiological studies
https://doi.org/10.15829/1728-8800-2021-2958
Abstract
The success and quality of large-scale epidemiological studies depends entirely on biomaterial quality. Therefore, when arranging the third Epidemiology of Cardiovascular Diseases and their Risk Factors in Regions of Russian Federation (ESSE-RF-3) study, increased attention was paid to specifics of collection, processing and further transportation of biological samples and related clinical and anthropometric data of participants from regional collection centers to Biobank.
Aim. To develop a methodology for collection of high-quality biomaterials within the large-scale epidemiological study, involving the sampling, processing, freezing of blood and its derivatives (serum, plasma) in the regions, followed by transportation and storage of obtained biomaterial in the Biobank of National Medical Research Center for Therapy and Preventive Medicine (Moscow).
Material and methods. To conduct the ESSE-RF-3 study, a design was developed, according to which the collection of venous blood samples in a total volume of 29,5 ml from each participant is planned in all participating regions in order to obtain and store samples of whole blood, serum and two types of plasma.
Results. On the basis of international biobanking standards, ethical norms, experience from ESSE-RF and ESSE-RF-2, and literature data, a protocol for biobanking of blood and its derivatives was developed. The type and number of serum and plasma aliquots obtained, the required standard technical means and consumables, as well as logistic biomaterial requirements were determined. Training programs for regional participants were developed. By the beginning of August 2021, 180 thousand samples of whole blood, serum and plasma from more than 23 thousand participants from 28 Russian regions were collected, processed and stored.
Conclusion. The presented work made it possible to assess and confirm the compliance of developed biobanking protocol with quality requirements. However, due to the coronavirus disease 2019 pandemic, by August 2021, the Biobank did not reach the maximum effectiveness predicted for the ESSE-RF-3 project.
For citations:
Pokrovskaya M.S., Borisova A.L., Metelskaya V.A., Efimova I.A., Doludin Yu.V., Kozlova V.A., Serebryanskaya Z.Z., Balanova Yu.A., Meshkov A.N., Pustelenin A.V., Imaeva A.E., Shalnova S.A., Kontsevaya A.V., Drapkina O.M. Role of biobanking in managing large-scale epidemiological studies. Cardiovascular Therapy and Prevention. 2021;20(5):2958. https://doi.org/10.15829/1728-8800-2021-2958
Introduction
The third Epidemiology of Cardiovascular Diseases and their Risk Factors in Regions of Russian Federation (ESSE-RF-3) study is a unique large-scale research project dedicated to the study of the prevalence of cardiovascular diseases and their risk factors in various Russian regions [1]. Within the framework of this project phase, a survey of representative samples of men and women aged 25-64 years (n=60 thousand), living in 30 Russian regions with different climatic and geographical characteristics, is carried out. The community-based sampling was carried out based on regional healthcare centers according to the Kish method [2].
ESSE-RF-3 is an epidemiological trial that studies the prevalence of risk factors for noncommunicable diseases on a national scale. Such studies, carried out on a regular basis, make it possible to create a system for health status monitoring of Russian population.
The ESSE-RF-3 study consists of 3 phases: 1) questionnaire, 2) instrumental investigations and 3) laboratory tests. The inclusion of biochemical parameters in the study allowed to analyze their associations with the socio-demographic and clinical characteristics of examined individuals. Laboratory tests were planned to be carried out in the clinical diagnostic laboratory of National Medical Research Center for Therapy and Preventive Medicine. To do this, it was necessary to collect blood and prepare sample in the regions, and then transport it to the Biobank National Medical Research Center for Therapy and Preventive Medicine. Obviously, the success of such a large-scale study depends entirely on the quality of the biomaterial used. Therefore, special attention was paid to the development of a methodology for collecting, processing and further transportation of biological samples from regional collection points to the above-mentioned biobank.
Biobank is a collection of biological material and related information stored in an organized system that can be used in population and clinical research at the present time or in the future [3][4]. The key difference between a biobank and research collections is the provision of responsible safe storage of biomaterial, strict adherence to ethical requirements and standards for the collection, processing, transportation and storage of biological samples, as well as the availability of patient/donor data.
The role of biobank in epidemiological research is very important. The use in research activities of biomaterial collected, processed and stored according to uniform rules and standards is a guarantee of the reliability, reproducibility and high quality of results obtained in current and future studies [5][6].
The most famous and striking example of organizing and conducting large-scale collection and longterm storage of biosamples and associated data is the UK Biobank [7][8], which collected blood, urine and saliva samples from 500000 Britons aged 40-69 years over 4 years. The uniqueness of the project lies in completeness and availability of data on biosamples for scientists around the world.
Biobank of the National Medical Research Center for Therapy and Preventive Medicine. For carrying out the planned laboratory studies within the ESSE-RF-3 project, as well as for long-term safe storage and ensuring high quality of biosamples and data collected at regional points, the biomaterial is transported to the Biobank of National Medical Research Center for Therapy and Preventive Medicine, organized and functioning in accordance with international standards.
This Biobank, established in 2014, is a center for the systematic collection and responsible storage of collections of high-quality biological samples and related clinical and laboratory information for conducting largescale epidemiological studies. The quality of received and stored biosamples and the data associated with them is confirmed by international quality certificate ISO 9001:2015, received by our Biobank. By the beginning of the ESSE-RF-3, the Biobank already stored the biomaterial collected during the first and second ESSERF stages (>20 thousand people) from 12 Russian regions, as well as whole blood, serum and plasma samples from 33 research projects. As of August 2021, the total number of biosamples is more than 459 thousand.
Biobank staff has accumulated experience in biobanking of blood for large-scale epidemiological studies (ESSE-RF, ESSE-RF-2), which made it possible to quickly develop an improved methodology for collecting and transporting biomaterial for a much largerscale stage of the project.
Safe storage of biosamples in the Biobank is provided by modern equipment, including low-temperature freezers, continuous monitoring systems, uninterruptible power supply, software for systematic and safe store of a large amount of biosample data. The quality of received and stored biosamples and associated data is confirmed by an international quality certificate ISO 9001:2015 “Quality management systems — Requirements”.
In preparation for the ESSE-RF-3 study, new Biobank premises intended for biomaterial storage and organized in accordance with the best world experience were commissioned.
All participants signed written informed consent, approved by the Ethics Committee of the National Medical Research Center for Therapy and Preventive Medicine. Depersonalization of personal data was carried out.
The aim was to develop a methodology for collection of high-quality biomaterials within the largescale epidemiological study, involving the sampling, processing, freezing of blood and its derivatives (serum, plasma) in the regions, followed by transportation and storage of obtained biomaterial in the Biobank of National Medical Research Center for Therapy and Preventive Medicine.
Material and methods
To collect biomaterial according to the ESSE-RF-3 research protocol on the basis of international biobanking standards, ethical standards, experience in carrying out ESSE-RF, ESSE-RF-2 projects and analysis of literature data [9-13], the Biobank staff developed a design for collecting venous blood samples in a total volume of 29,5 ml from each participant in order to obtain and store samples of whole blood, serum and two types of blood plasma in all regions participating in the study.
The design defines the type and number of serum and plasma aliquots (Table 1). The calculation was carried out in such a way as to cover the widest possible range of potential studies on this biomaterial in current and future projects. The number of aliquots from each participant in the study was determined based on the type and number of planned analyzes, as well as on the need of a single use of biological sample without additional freeze/thaw cycles. Additionally, in twelve of thirty regions, the study design included analysis of glycated hemoglobin (HbA1c) levels.
Table 1
Type and number of aliquots received within ESSE-RF-3 project
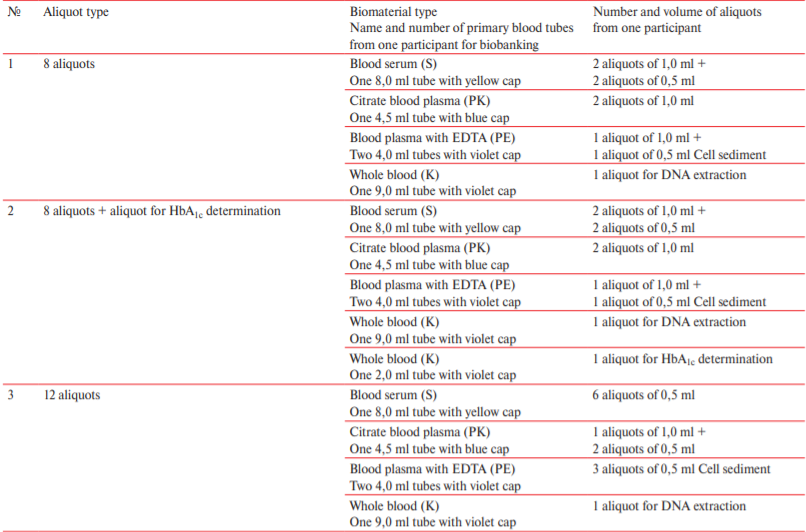
Note: DNA — deoxyribonucleic acid, EDTA — ethylenediaminetetraacetic acid, HbA1c — glycated hemoglobin.
For safe and responsible storage of biomaterials and information support, Biobank freezers and the FreezerPRO program were used.
Informed consent with the participant’s identification number instead of personal data, signed by all ESSE-RF-3 participants in duplicate, are stored in a special storage facility of Biobank.
Results and discussion
According to the study design, a methodology for collecting, processing, transporting and storing biological samples was developed. Standard operating procedures (SOPs) describe in detail the processes of collection, processing of biomaterial, registration in the data entry program, temperature regimes and exposure times of biological samples before processing and during storage, preparation of biomaterial for transportation to Biobank, as well as the exporting data from program and form, which must be filled in order to form the database. A platform and a program for registering biological samples and forming a database have been created. Strict adherence to this regulation and the use of developed software complies with biobanking standards and ensures the high quality of biomaterial and information support.
For all regions participating in the study, a standard set of equipment and consumables required for obtaining biosamples was determined. The use of this approach reduces the risks of influence of technical characteristics and settings of various equipment on biosample quality. For example, when using freezers with different temperature conditions, the sample freezing time may differ, while the use of a single set of equipment and consumables allows to manage work according to a single standard, including SOPs for each of the stages and video instructions.
A standard set of equipment and consumables for each point for collection, processing and temporary storage of biosamples includes the following components: a refrigerated medium speed laboratory centrifuge, a freezer with a temperature of -250C, a computer with preinstalled software and Internet access, barcode scanner, variable volume pipettes with disposable tips, vacuum blood collection systems, racks and 2D barcode cryogenic vials.
The key factor affecting the quality of biosamples, the availability, completeness and reliability of related data is the accuracy of instructions execution. Biobank staff developed demonstration materials, including instructions, a presentation and a video describing in detail all the actions of employees of regional health care facilities, from the procedure for blood collection and ending with the preparation of biomaterial and accompanying documentation for transportation. In each region, training was carried out for project executors using these materials. In addition, the regional contractors continue to provide prompt and regular online consultations on all emerging issues of collection, preparation and transportation of biomaterial according to biobanking standards.
Main biobanking stages
Biomaterial collection. Only certified medical personnel are allowed to work with vacuum blood collection systems. Peripheral (venous) blood samples should be collected aseptically using sterile needles and adapters in BD Vacutainer tubes labeled with unique sample indexes.
The study participants must follow a following number of general recommendations: on the eve of blood sampling, physical activity, stress, physiotherapy procedures should be excluded; medications (decision to discontinue drug treatment is made by the attending physician), oral contraceptives, alcohol and fatty foods should not be used. Smoking should be avoided for 1 hour immediately prior to sampling. In the treatment room of healthcare facility, a standard blood sample is taken in the morning on an empty stomach at rest from the cubital vein.
Before the blood sampling procedure, the nurse provides the following elements:
1. Separates from the roll the labels with the 6-digit serial number of a participant (Figure 1), which contain the following information: upper line — name of the region, lower line — 6-digit number of a participant XXNNNN (XX — number of the region according to the Russian Classification on Objects of Administrative Division, NNNN — a participant’s serial number) and the type of biological sample that must be obtained from this tube (S, PK, K) (Table 1, Figure 1). The single participant label kit also includes a direction label.

Figure 1. Primary tube label set and appointment card for one participant.
2. Fills in the appointment card (Figure 2): Full name of a participant and a unique 8-digit identification number, sticks a label with a 6-digit serial number, records the time of blood collection.

Figure 2. Appointment card form.
A unique 8-digit identification number is assigned to each participant at the initial study stage when sampling using the Kish method. However, the use of only this code in their work creates a number of difficulties, since some of the respondents included may refuse to participate in the study. In addition, the participants come to donate blood inconsistently, which leads to difficulties in finding the necessary labels for labeling the tubes. To simplify the work of medical workers, a system of 6-digit sequential numbers was additionally introduced, assigned to each participant who came to the healthcare center.
3. Adheres labels to blood collection tubes: one 8,0 ml serum tube with yellow cap (labeled S), one 9,0 ml tube with ethylenediaminetetraacetic acid (EDTA) with purple cap for whole blood (labeled K), two 4,0 ml tubes with EDTA with violet cap for whole blood and EDTA plasma (labeled K), one 4,5 ml tube with sodium citrate with a blue cap for plasma with sodium citrate (labeled PK) (Figure 3).

Figure 3. Primary blood collection tube set for one study participant.
Ethical requirements governing the biobanking do not allow the personal data of participants (full name) to be applied to test tubes. The 8-digit identification number of a participant from the registration card is entered in the appointment card, and labels with a 6-digit number corresponding to the serial number of a participant are glued to test tubes. The 6-digit number is linked to an 8-digit identification number in the computer program used to register biosamples. In this way, the data is depersonalized. The doctors fill in the so-called conversion tables, which contain the personal data of participants and their corresponding identification numbers. Neither the staff carrying out the sample preparation, nor the staff of the National Medical Research Center for Therapy and Preventive Medicine, have access to the personal data of participants.
The sampling sequence is of great importance: yellow cap tubes are first filled, then — blue ones, and at the end — purple ones. Immediately after blood collection, the contents of tubes with purple and blue caps are mixed, gently inverting five times, and placed in a rack in an upright position. Tubes should not be shaken as it can lead to hemolysis. Contents of tubes with yellow caps (not containing anticoagulant) is not mixed.
At the end of procedure, the nurse checks the completed appointment card and gives the tubes and appointment card to the laboratory technician for sample preparation.
Sample preparation − biological material processing after blood collection. The sample preparation procedure consists of blood centrifugation and sample aliquoting. All tubes are centrifuged 30 minutes after blood collection. It is important not to lengthen the time before centrifugation for sodium citrate tubes (blue caps) to obtain blood plasma for coagulation studies. The time to centrifugation of tubes with yellow and purple caps can be up to 1 hour. It is worth noting that 9,0 ml and 4,0 ml tubes with a purple cap for freezing whole blood are not centrifuged.
The tubes are centrifuged at a temperature of +40C at a speed of 1900 g (3260 (rpm) for 15 minutes). At the end of procedure, it is necessary to make sure that the serum/plasma is clearly separated from the cellular components and there are no hemolysis. Cellular components should not be above the sediment. If there is a problem with serum/plasma separation or clotting, recentrifugation is performed. In the case of hemolyzed or chylous serum/plasma, blood collection is repeated.
The resulting serum and plasma samples are aliquoted into cryovials containing factory unique linear barcodes on the side and 2D barcodes on the bottom (Figure 4). This barcode of the cryovial is connected to the 8-digit identification number of a participant already entered into the database. In these tubes, serum and blood plasma are transported and stored.

Figure 4. Cryotubes with a unique 2D barcode for long-term storage of serum and plasma samples.
Aliquots of biosamples from tubes with yellow, blue and violet caps are performed with an automatic dispenser using disposable tips with a volume of 100- 1000 μL. It is important to collect only pure serum and plasma without mixing with a blood clot and without touching the tip to sediment layer. After aliquoting each type of biosample, the tip must be changed.
When aliquoting EDTA plasma from 4,0 ml violet cap tubes, a 1 mm layer of plasma above the sediment should be left. This cell pellet tube should be frozen along with two other violet cap tubes containing whole blood with EDTA. These biosamples will be used for genetic research.
During sample preparation, it is important to record the time of the beginning of centrifugation and aliquoting, as well as the number and volume of aliquots obtained in “Information on biosamples” list (Figure 5). By filling out this form, the accuracy of further work with the program increases, and the necessary information is duplicated on paper.

Figure 5. “Information on biosamples” list form.
Note: S — blood serum, PE — blood plasma with EDTA, PK — citrate blood plasma.
Data entry program. Information about the collected biomaterial is entered and stored in the ESSERF-3 project database. Information about biological samples is entered using a special ESSE-RF-3 program. The developed IT platform during the ESSE-RF-2 project was significantly improved for the ESSE-RF-3. It allows to register and track information on the processing of biosamples and related data online. This was an important step in preparing for the study. The program provides the use of so-called input mask (Figure 6). The following information is entered into the program: an 8-digit participant identification number at initial registration, then scanned barcodes of all types of samples received, the name and number of region, the full name of an employee performing the sample preparation, the date and time of blood sampling, the time of centrifugation start, the time of freezing, and the status of sending from the region to Biobank. The data is stored in a depersonalized form, which meets all the ethical.

Figure 6. The interface of program for entering and storing data related with biosamples.
Information about biosample type is entered by scanning the barcodes located on cryovials and on the labels of vacutainers with whole blood and cell sediment into data entry program as follows: K — for whole blood samples, S — for serum, PK — for citrate plasma, PE — for plasma with EDTA (Figure 7).

Figure 7. Window for entering data encoded in cryotube barcodes.
Additionally, the program allows to generate lists of biosamples in table form in Excel format.
Freezing. After entering the data into the ESSERF-3 program, cryo stands with cryovials and test tubes with whole blood and cell sediment in a freezing stand are placed in a freezer with a storage temperature of -250C. At this temperature, samples should be stored no longer than 3 weeks, since, according to numerous studies, blood serum storage during this time period does not significantly affect its quality [14][15]. Further, biosamples should be transported to the Biobank for storage at -700С.
At the end of each working day, the frozen violet cap tubes are collected in a zip bag marked with the current date. In the same zip package, the “Information list” of current day is included.
Transfer of samples to Biobank. Transportation of samples from regional collection points, sample preparation and freezing of biomaterial to Biobank is carried out by a transport company once every 3 weeks in containers with dry ice at temperatures from -50 to -700C using temperature data loggers, which allow continuous monitoring of the temperature inside the transport box at all stages of transportation.
During transportation, biological samples are accompanied by a following package of documents:
- filled in sheets “Information on biosamples”;
- informed consents signed by the participants (2 copies);
- reports of acceptance/transfer of biomaterial, containing information on the type and number of transferred biosamples.
Preparing for sending of biomaterial from the regional collection center to Biobank. The management of biomaterial transportation from the regional collection centers to Biobank is carried out by a specialist manager of Biobank, who have following duties: tracking the storage times of biosamples in health care centers, observing the time for sending of biomaterials from the moment of collection and then every 3 weeks, communication with the regional manager, obtaining information on the amount of biomaterial ready for sending, agreeing with the transport company on the scope of delivery and time of departure from the collection center, as well as the time of arrival at Biobank. At the same time, the regional project manager prepares a file with a list of numbers of biosamples ready to be sent from the data entry program and sends it by e-mail to Biobank.
On the agreed day and time, the regional project managers, together with the courier of transport company, move the biosamples in racks from the freezer to transport bag and into the transport container with dry ice, where they also place the temperature logger and zip bags with test tubes containing the whole blood and cell sediment.
“Information on biosamples” lists, informed consent and reports of acceptance/transfer are packed in a certain package.
Transportation of biomaterial to Biobank is carried out in compliance with the times and temperature regimes and monitoring of cold chain (dry ice, temperature not higher than -500С).
Transfer of biological samples within a region. If the center where the collection, sample preparation of biomaterial and data input into the program is carried out is outside the direct access to the freezer at -250C, the biomaterial after sample preparation according to the instructions without preliminary freezing is delivered at a temperature from +4 to +80C to the freezer. The holding time at this temperature before freezing in a freezer at -250C should not exceed 3 hours from the centrifugation time. For transportation, a cooler bag with ice packs is used.
Acceptance of biomaterial sending from the regions to Biobank. In Biobank, registration of biomaterial transported from regions is carried out as follows:
1) scanning of racks with serum and plasma samples, as well as tubes with whole blood and cell sediment;
2) export of CSV files containing information about biosamples;
3) checking and editing the received files with the introduction of data on placement of accepted biosamples for long-term safe storage in freezers: whole blood and cell sediment at -300C, serum and blood plasma at -700C;
4) adding the edited files to the FreezerPRO program.
To carry out the laboratory tests, Biobank transfers serum samples to the laboratory of National Medical Research Center for Therapy and Preventive Medicine. Research results are automatically entered into the ESSE-RF-3 database and become available in the regions for issuance to participants. If additional studies using stored biological samples are necessary, the Biobank issues aliquots of the biomaterial according to certain regulations.
By the beginning of August 2021, 180 thousand samples of whole blood, serum and plasma from more than 23 thousand participants from 28 Russian regions were collected, processed and placed for storage. The pandemic of coronavirus disease 2019 (COVID-19) had a significant negative impact on the speed of project implementation, which led to a slowdown in regional collection of biomaterial. In this regard, at present, the Biobank work has not reached the maximum performance predicted for the ESSE-RF-3 project. However, the work carried out has already made it possible to assess and confirm the compliance of developed biobanking protocol with the requirements for the quality of obtained biosamples and related data.
It is worth noting the importance of the inf luence of human factor on the study. Within the ISO 9001:2015 “Quality management systems — Requirements” standard operating in the Biobank, special attention is paid to the discrepancy management. Their timely identification and informing the staff of regional health care center allow them to quickly develop corrective measures, control the implementation of each biobanking stage, reduce the risk of repeated errors and, as a result, significantly improve the quality of obtained biosamples and related data [3][16].
Thus, the developed and implemented methodology for the collection, preparation, transportation and storage of biological samples within the ESSERF-3, exceeding all previous epidemiological studies in Russia, can be considered as a universal tool for obtaining and storing high-quality human biological material.
Conclusion
In conclusion, it should be noted that the successful implementation of large-scale epidemiological projects involving the collection, processing, transportation and long-term storage of a large amount of biomaterial and data from numerous Russian regions requires careful planning and an integrated approach to the management, including the creation of protocols for all stages of work on a single standard:
- determination of the type and amount of collected biomaterial;
- selection of equipment and consumables to ensure high quality and safety of obtaining and storing biosamples at all project stages, including for a long time;
- development of forms and SOPs for all stages of collection, preparation and transportation of biomaterial from regions to Biobank;
- development of software and databases for input, transmission and long-term storage of related data;
- selection of a reliable logistics company capable of ensuring and documenting compliance with the required time and temperature conditions for transportation;
- personal and distance learning of regional project performers using the developed instructions and prepared presentation materials, including in videos recording all processes related to blood collection, processing of biosamples, freezing and preparation for transportation.
Obviously, when organizing large-scale epidemiological studies at the present stage, the Biobank is a necessary central link. It is the biobanking regulations and the centralized management of biomaterial collection that ensure the high quality of collected biomaterial, which is necessary to obtain reliable and reproducible research results. With a unified regulation, Biobank collects a unique structured collection of biomaterials from a representative sample of population from 30 Russian regions for research within the ESSERF-3 and for future research projects.
On the basis of international biobanking standards, ethical norms, experience from ESSE-RF and ESSERF-2, and literature data, a protocol for biobanking of blood and its derivatives was developed, which can be used for large-scale research projects.
Despite the fact that currently the Biobank work has not reached the peak performance predicted for the ESSE-RF-3 project due to COVID-19 pandemic, the work carried out made it possible to assess and confirm the compliance of developed biobanking protocol with the requirements for the quality of obtained biosamples and related data.
The biomaterial collected within the study potentially makes it possible to carry out a wide range of metabolomic, proteomic and genomic studies in the future.
Relationships and Activities: none.
References
1. Scientific Organizing Committee of the ESSE-RF. Epidemiology of cardiovascular diseases in different regions of Russia (ESSE-RF). The rationale for and design of the study. Profilakticheskaya Meditsina. 2013;16(6):25-34. (In Russ.)
2. Kish L. Survey Sampling. New York: John Wiley and Sons, 1965. 643 p. ISBN: 047148900X (ISBN13: 9780471489009).
3. Pokrovskaya MS, Borisova AL, Sivakova OV, et al. Quality management in biobank. World tendencies and experience of biobank of FSI “NMRC for Preventive Medicine” of the Ministry of Healthcare of Russia. Klinicheskaya Laboratornaya Diagnostika [Russian Clinical Laboratory Diagnostics]. 2019;64(6):380-4. (In Russ.)
4. Reznik ON, Kuzmin DO, Skvortsov AE, et al. Biobanks are an essential tool for transplantation. History, current state, perspectives. Russian Journal of Transplantology and Artificial Organs. 2016;18(4):123-32. (In Russ.)
5. Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery. 2011;10(9):712. doi:10.1038/nrd3439-c1.
6. Problems with scientific research: How science goes wrong. The Economist. 2013;Oct, 19th. https://www.economist.com/leaders/2013/10/21/how-science-goes-wrong.
7. Elliott P, Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. International J Epidemiol. 2008;37(2):234-44. doi:10.1093/ije/dym276.
8. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med. 2015;12(3):e1001779. doi:10.1371/journal.pmed.1001779.
9. Anisimov SV, Glotov AS, Granstrem OK, et al. Biobanks and biomedical progress. Saint-Petersburg: Svoe izdatel'stvo. 2018, 86 p. (In Russ.)
10. Doludin YV, Borisova AL, Pokrovskaya MS, et al. Current best practices and biobanking recommendations. Klinicheskaya Laboratornaya Diagnostika [Russian Clinical Laboratory Diagnostics]. 2019;64(12):769-76. (In Russ.)
11. Pokrovskaya MS, Sivakova OV, Meshkov AN, et al. Organization of biobanking of biological samples within the second stage of epidemiological study of cardiovascular risk factors and diseases in the regions of the Russian Federation (ESSE-RF2). The Russian Journal of Preventive Medicine. 2018;21(2-2):44-5. (In Russ.)
12. Iacoviello L, Curtis AD, Donati MB, et al. Biobanks for cardiovascular epidemiology and prevention. Future Cardiol. 2014;10(2):243-54. doi:10.2217/fca.13.110.
13. ISO 20387:2018 Biotechnology — Biobanking — General requirements for biobanking. https://www.iso.org/standard/67888.html.
14. Kozlova VA, Metelskaya VA, Pokrovskaya MS, et al. Stability of serum biochemical markers during standard long-term storage and with a single thawing. Cardiovascular Therapy and Prevention. 2020;19(6):2736. (In Russ.)
15. Sivakova OV, Pokrovskaya MS, Efimova IA, et al. Quality control of serum and plasma samples for scientific research. The Russian Journal of Preventive medicine. 2019;22(5):91-7. (In Russ.)
16. ISO 9001:2015 Quality management systems — Requirements. https://www.iso.org/standard/62085.html.
About the Authors
M. S. PokrovskayaRussian Federation
Moscow.
Tel.: +7 (965) 225-55-27
A. L. Borisova
Russian Federation
Moscow.
V. A. Metelskaya
Russian Federation
Moscow.
I. A. Efimova
Russian Federation
Moscow.
Yu. V. Doludin
Russian Federation
Moscow.
V. A. Kozlova
Russian Federation
Moscow.
Z. Z. Serebryanskaya
Russian Federation
Moscow.
Yu. A. Balanova
Russian Federation
Moscow.
A. N. Meshkov
Russian Federation
Moscow.
A. V. Pustelenin
Russian Federation
Moscow.
A. E. Imaeva
Russian Federation
Moscow.
S. A. Shalnova
Russian Federation
Moscow.
A. V. Kontsevaya
Russian Federation
Moscow.
O. M. Drapkina
Russian Federation
Moscow.
Supplementary files
Review
For citations:
Pokrovskaya M.S., Borisova A.L., Metelskaya V.A., Efimova I.A., Doludin Yu.V., Kozlova V.A., Serebryanskaya Z.Z., Balanova Yu.A., Meshkov A.N., Pustelenin A.V., Imaeva A.E., Shalnova S.A., Kontsevaya A.V., Drapkina O.M. Role of biobanking in managing large-scale epidemiological studies. Cardiovascular Therapy and Prevention. 2021;20(5):2958. https://doi.org/10.15829/1728-8800-2021-2958

























































