Scroll to:
Clinical and anamnestic characteristics of patients with non-ST elevation myocardial infarction after COVID-19
https://doi.org/10.15829/1728-8800-2021-3062
Abstract
Aim. To study clinical, medical history and paraclinical characteristics of patients with non-ST elevation myocardial infarction (NSTEMI) after coronavirus disease 2019 (COVID-19).
Material and methods. The study included 209 patients with NSTEMI who were admitted to the Demikhov City Clinical Hospital (Moscow). The patients were divided into 2 groups: the experimental one (n=104) — those after COVID-19, the control one (n=105) — those without history of COVID-19. All patients underwent routine diagnostic investigations in accordance with current standards and clinical guidelines.
Results. The mean age of patients in the experimental group was 61,8±12,2 years, while in the control one — 69,0±13,0 years (p<0,0001). Myocardial infarction developed 49 days [34.0; 82.0] after COVID-19. Prior exertional angina was observed in 76,9% of patients in the experimental group and in 88,6% in the control one (χ2 =4,97; p=0,0258). The level of C-reactive protein in the experimental group was 19,2 mg/l [4,9; 53,0], and in the control one — 5,6 mg/l [0,4; 21,8] (p=0,0007). The average troponin I level in the experimental group was 2,7 ng/ml [1,3; 8,0], while in the control one — 1,8 ng/ml [0,8; 3,5] (p=0,0091).
Conclusion. Patients with NSTEMI after COVID-19 were significantly younger compared to patients without a history of COVID-19. They had less common exertional angina prior to MI, while C-reactive protein and troponin I levels were significantly higher than in the control group. In addition, in NSTEMI patients after COVID-19, the estimated pulmonary artery systolic pressure was significantly higher compared to patients without a history of COVID-19.
For citations:
Chashchin M.G., Gorshkov A.Yu., Drapkina O.M., Kositsyna I.V., Golubev A.V., Chaus N.I., Perekhodov S.N. Clinical and anamnestic characteristics of patients with non-ST elevation myocardial infarction after COVID-19. Cardiovascular Therapy and Prevention. 2021;20(7):3062. https://doi.org/10.15829/1728-8800-2021-3062
Introduction
In December 2019, an outbreak of a coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in the People’s Republic of China, which quickly acquired the scale of a pandemic. At the time of writing, according to Johns Hopkins Coronavirus Resource Center, the total number of detected COVID-19 cases has reached 222,5 million with 4,5 million deaths [1]. Despite the ability of COVID-19 to cause severe acute respiratory syndrome in some patients, in most cases the symptoms are mild or absent and have not significant clinical manifestations [2]. However, regardless of the disease’s course, published data indicate a long-term coagulation changes in the post COVID-19 period and an increased level of inflammatory markers in survivors [3].
Greenhalgh Т suggested define post-acute COVID-19 as extending beyond three weeks, and chronic COVID-19 as extending persisting symptoms beyond 12 weeks from the onset of first symptoms [4]. At the end of 2020, the COVID-19 Rapid Guideline [5] first introduced terms and definitions of time intervals dividing post-COVID-19 period into the acute COVID-19 — signs and symptoms of COVID-19 for up to 4 weeks; ongoing symptomatic COVID-19 — signs and symptoms of COVID-19 from 4 to 12 weeks; post-COVID-19 syndrome — signs and symptoms that develop during or after an infection SARS-CoV-2, continue for more than 12 weeks and are not explained by an alternative diagnosis; long-term COVID — is an umbrella term for describing the clinical condition that developed in the acute COVID-19 period and did not regress after 4 weeks of diseases. It is believed that the long-term effects of COVID-19 do not correlate with the severity of acute infection, and the possible long-term consequences at the moment remain only hypothetical [3].
Cardiovascular patients constitute a special group. Comorbid cardiovascular disease suggests both high risks of cardiovascular events (CVEs) and severe COVID-19. Hypoxemia, endothelial dysfunction, systemic inflammation, progression of atherosclerosis, prothrombotic activation of the hemostasis system can lead to acute coronary syndrome (ACS) both in the acute COVID-19 and post-COVID-19 periods [6].
Recent studies on influenza infection confirm the high risks of CVD among survivors [7][8]. However, data indicating a direct relationship between prior viral infections (including COVID-19) and their contribution to the development of myocardial infarction (MI) are currently limited. Persistent inflammation in postCOVID-19 period, indicated by elevated levels of C-reactive protein (CRP) and interleukin-6, may predispose to ACS and affect the severity of MI [9][10].
The aim was to study clinical, medical history and paraclinical characteristics of patients with nonST elevation myocardial infarction (NSTEMI) after COVID-19.
Materials and methods
The study consecutively included 209 patients who were treated from July 2020 to March 2021 in the intensive cardiac care unit of the Demikhov City Clinical Hospital (Moscow) with established NSTEMI. All patients underwent a full range of diagnostic and therapeutic measures in accordance with the current standards, as well as current clinical guidelines for patients with NSTEMI of the Ministry of Health of Russia, including coronary angiography and stenting when necessary.
There were following inclusion criteria: clinically and paraclinically confirmed diagnosis of NSTEMI, angiographic signs of obstructive coronary artery disease, the absence of clinical manifestations of acute respiratory viral infections at the time of admission, age >18 years.
There were exclusion criteria as follows: acute myocardial injury due to percutaneous coronary intervention (PCI) or other surgical treatments; clinical or laboratory evidence of the acute COVID-19 at the time of admission, or identified during hospitalization.
Prior COVID-19 was established by anamnestic data, discharge summary (with positive polymerase chain reaction (PCR) test on COVID-19 or the presence of anti-SARSCoV-2 IgG antibodies, characteristic chest computed tomography abnormalities). Asymptomatic cases were defined as a significant increase in anti-SARS-CoV-2 IgG antibodies (> upper reference value), without clinical manifestations of COVID-19.
The diagnosis of MI was made in accordance with the fourth universal definition of MI, according to 2018 European Society of Cardiology guidelines [11].
At admission, a detailed medical history was collected and an objective examination was carried out. In addition, nasopharyngeal and oropharyngeal swabs were taken to diagnose COVID-19 using PCR test, the titer of anti-SARSCoV-2 IgM and IgG antibodies was determined. Complete blood count and biochemical profile was assessed, including definition of the CRP level, cardiac troponin I, coagulation tests; 12-lead electrocardiography (ECG), echocardiography and coronary angiography were performed.
The investigation of anti-SARS-CoV-2 antibodies was performed by a semi-quantitative method on the CL 6000i analyzer (Shenzhen Mindray Bio-Medical Electronics Co.; China) with reference values for IgM up to 2 U/ml and for IgG up to 10 U/ml.
Statistical processing was carried out using Microsoft Excel, IBM SPSS Statistica 26 (USA) application packages. Mean and standard deviation (M±SD) were used to describe normally distributed quantitative variables, and the median and interquartile range (Me [Q25; Q75]) were used for nonnormal distributions. The normality of distribution was tested using the Kolmogorov-Smirnov test. Qualitative variables are described as an absolute number and frequency of trait detection n (%). Differences between two independent groups for continuous variables were determined using the MannWhitney U-test. The statistical significance of differences between qualitative variables was determined using Pearson’s χ2 test or Fisher’s exact test. Differences were considered significant at p<0,05.
Results
The mean age of patients in the general group was 65,4±13,1 years. More than half of participants were men (55%; n=115), while 18,2% (n=38) had a positive family history of early cardiovascular diseases in the first-degree relative. A significant part of the surveyed (82,8%; n=173) had effort angina before reference NSTEMI; 35,4% (n=74) of patients had prior MI. Previously, coronary angiography was performed in 24,4% (n=51), coronary artery stenting — in 20,1% (n=42), and coronary artery bypass grafting — in 1,4% (n=3); 31,6% (n=66) of patients were smokers, while 61,7% (n=129) had obesity. The vast majority of patients 78,9% (n=165) had hypertension, 23% (n=48) — dyslipidemia, 30,1% (n=63) — type 2 diabetes, 18,7% (n=39) — atrial fibrillation, 6,7% (n=14) — asthma, 19,6% (n=41) — chronic obstructive pulmonary disease, while 11,5% (n=24) of patients had prior stroke. COVID-19 was verified in 49,7% (n=104) of patients by anamnestic and laboratory tests. The median time from the onset of clinical manifestations of COVID-19 to the development of ACS, on average, was 49 days [ 34,0; 82,0 ]. The main data on the COVID-19 course and results of serological testing in the group of patients with NSTEMI are presented in Table 1. It should be noted that 74,1% (n=77) of patients had mild or asymptomatic COVID-19.
All patients included in the study were divided into two groups depending on the history of COVID-19: the experimental group consisted of 104 patients after COVID-19, and the control group consisted of 105 patients without prior COVID-19. The comparison results of clinical and anamnestic data of NSTEMI patients of the main and control groups are presented in Table 2. Patients of the experimental group were significantly younger than those from the control one. Among them, there were less persons with prior manifestations of angina pectoris. The proportion of women in the experimental group was 51% (n=53), in the control group — 39% (n=41), but the differences did not reach statistical significance (p=0,0834).
Table 3 presents the distribution of NSTEMI patients depending on the chronic kidney disease (CKD) stages. Among the control group patients, stage ≥3a CKD was significantly more common (χ2=15,44; df=4; p=0,0039). According to the distribution of other concomitant diseases and risk factors, patients of both groups were comparable.
Clinical and paraclinical data in both groups are presented in Table 4. Upon admission a typical chest pain was observed in the vast majority of both patient groups. The complaint on prior shortness of breath from regular activity had ~1/3 patients of the experimental and control groups (p=0,8449). Cough was often observed in experimental group of patients, however, the differences were not significant (p=0,0951). Hemodynamic parameters in both groups were comparable, and the average values did not deviate from generally accepted standards. According to the severity of acute heart failure (Killip), the experimental and control groups also did not differ from each other (p>0,05).
Table 5 presents the results of comparing echocardiography parameters in patients of both groups. Patients of the experimental group had a higher calculated high systolic pulmonary artery systolic pressure (PASP) than in the control group (p=0,0097). For other indicators, there were no significant differences between the groups (p>0,05).
The coronary angiography (Table 6) showed that both groups were also comparable in the severity of hemodynamically significant coronary involvement.
Laboratory data of both groups are presented in Table 7. No significant differences were found in the studied groups in complete blood count and coagulation tests (p>0,05). The biochemical profile of the experimental group of patients, showed significantly lower urea and creatinine level, while creatinine clearance was significantly higher than in the control group. The CRP and troponin I levels in the experimental group was significantly higher compared to the control group.
Table 1
Anamnestic and laboratory data of NSTEMI patients after COVID-19
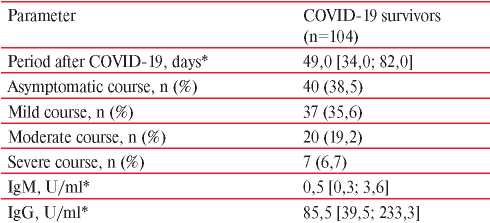
Note: * — Me [Q25; Q75].
Table 2
Comparison of the main clinical and anamnestic characteristics of NSTEMI patients depending on prior COVID-19
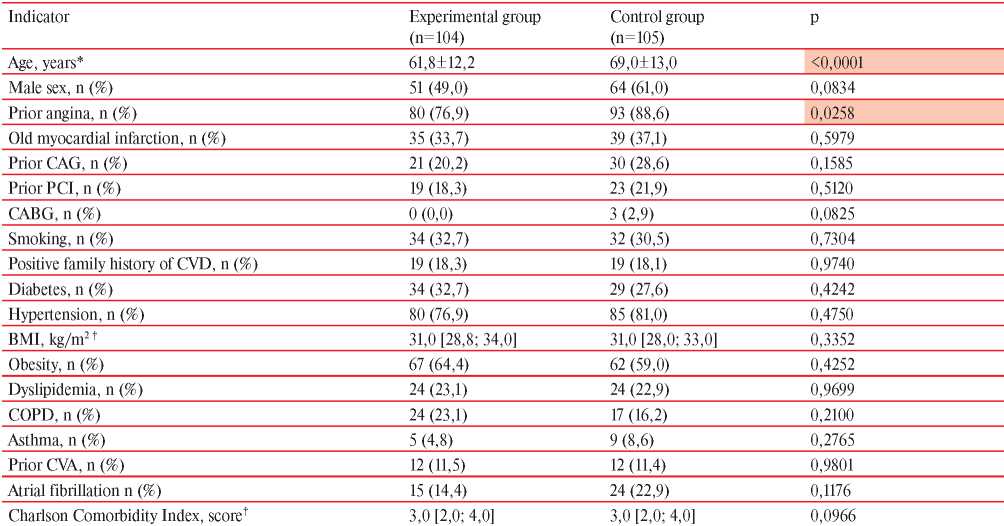
Note: CABG — coronary artery bypass grafting, BMI — body mass index, CVA — cerebrovascular accident, COPD — chronic obstructive pulmonary disease, PCI — percutaneous coronary intervention; * — M±SD; † — Me [Q25; Q75].
Table 3
Analysis of the distribution of NSTEMI patients depending on CKD stage
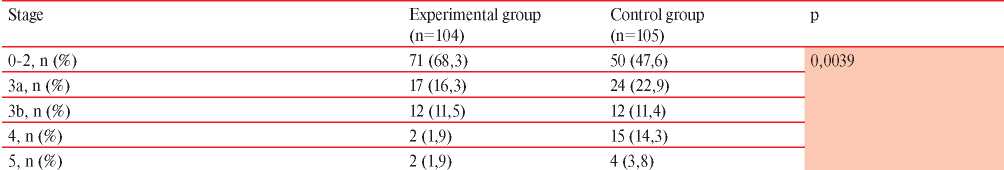
Table 4
Clinical data of patients with NSTEMI at hospital admission
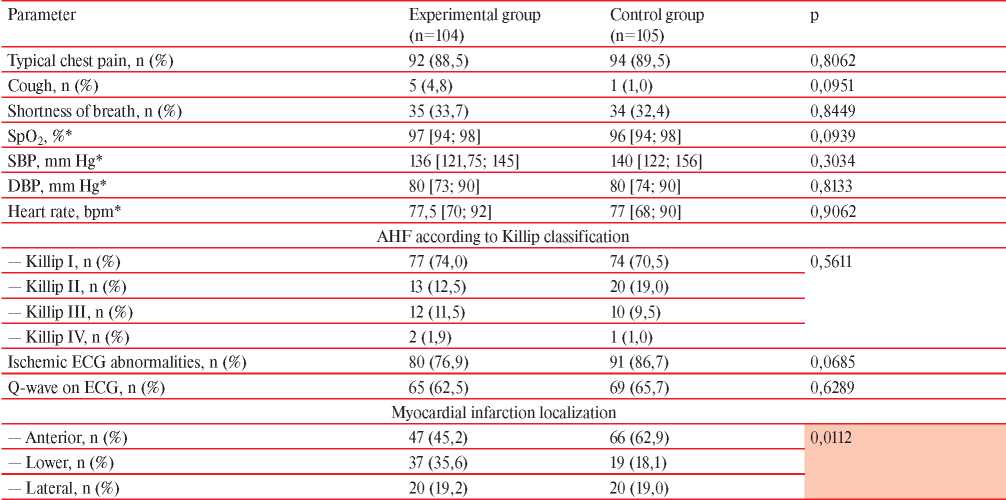
Note: DBP — diastolic blood pressure, AHF — acute heart failure, SBP — systolic blood pressure, HR — heart rate, * — Me [Q25; Q75].
Table 5
Echocardiographic characteristics of patients with NSTEMI
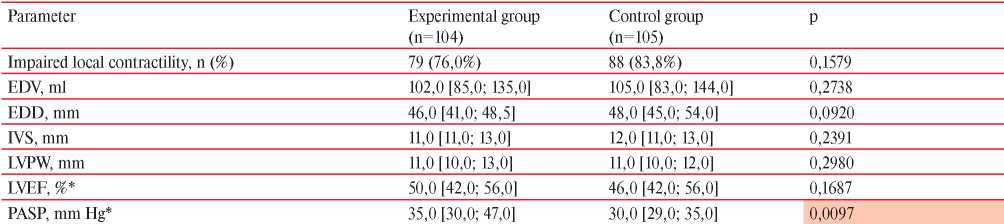
Note: EDV — end-diastolic volume, EDD — end-diastolic dimension, IVS — interventricular septum, LVPW — left ventricular posterior wall, LVEF — left ventricular ejection fraction, * — Me [Q25; Q75].
Table 6
Angiographic characteristics of coronary artery disease in NSTEMI patients
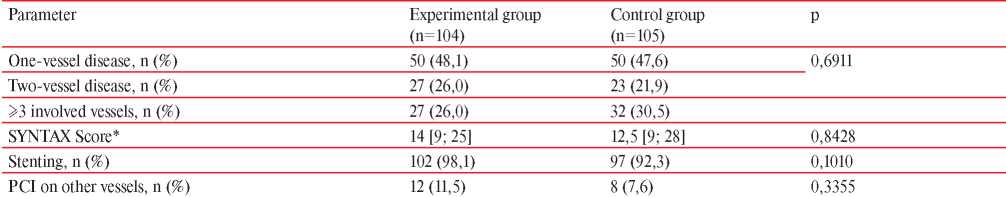
Note: SYNTAX Score — Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery; * — Me [Q25; Q75].
Table 7
Laboratory findings in NSTEMI patients
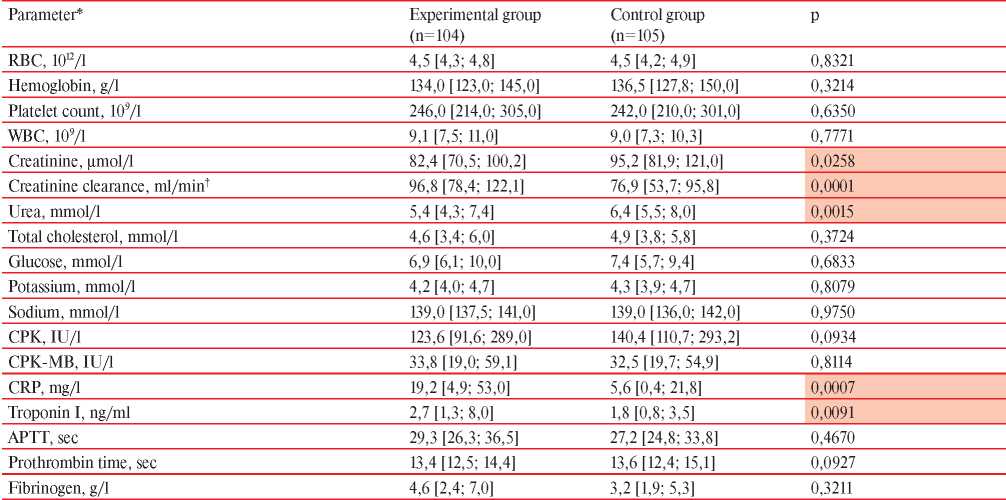
Note: APTT — activated partial thromboplastin time, CPK — creatine phosphokinase, CRP — С-reactive protein; * — Me [Q25; Q75]; †— according to the Cockcroft-Gault equiation.
Discussion
The presented analysis established a number of differences between NSTEMI patients with/without prior COVID-19 according to clinical, anamnestic, and paraclinical data. Patients with NSTEMI after COVID-19 were significantly younger compared to the control group. Despite the unreached statistical significance, there was a trend towards female predominance in the COVID-19 group compared with the control group. This may be due to a slightly higher incidence of infection among women, which, according to data published by the United Nations and the World Health Organization, is primarily due to socioeconomic factors [12]. Angina before MI was noted in the vast majority of patients in both groups. However, in the experimental group, its prevalence was slightly lower, which may be due to the younger age of patients and the predominance of female sex. The higher incidence of inferior NSTEMI observed in the experimental group may also be due to female predominance in the COVID-19 group. These data are comparable to previously published ones, which indicate a higher prevalence of inferior and lateral MI among women [13]. In patients after COVID-19, significantly higher values of PASP were recorded compared to the control group. These data are confirmed by already published research results, which indicate a high prevalence of echocardiographic signs of pulmonary hypertension, including increased PASP among patients with prior COVID-19 [14].
Of particular interest is the distribution of COVID-19 survivors according to its severity. According to the literature data, the incidence of asymptomatic COVID-19 is quite variable, and unambiguous epidemiological data have not yet been presented. In most articles, the prevalence of asymptomatic COVID-19 is 35-40% [15], but according to some data it can exceed 80%. The population study conducted during COVID-19 epidemic wave in St. Petersburg recorded asymptomatic course in 84,5% of cases among seropositive individuals (n=2713) without prior COVID-19 and a positive PCR test [16]. Similar results were demonstrated in the report by Ing A [17] on the spread of COVID-19 in the isolated Ernest Shackleton cruise ship, which had 217 passengers on board (81% had asymptomatic COVID-19), while >50% of Diamond Princess passengers also had an asymptomatic course [18]. When comparing the data obtained with previously published, it is noteworthy that in 61,5% of cases, NSTEMI patients had a symptomatic COVID-19, which makes it possible to consider this category as the most at CVD risk in post-COVID-19 period. In addition, 74,1% of patients experienced asymptomatic or mild COVID-19, which causes significant concern and requires further examination and monitoring of patients after COVID-19, regardless of its severity.
Patients after COVID-19 had lower levels of creatinine and urea and high creatinine clearance, which is a consequence of the relatively low prevalence of CKD in COVID-19 survivors as a whole. In addition, post-COVID-19 patients had higher CRP levels than patients without prior COVID-19. Published reports of studying post-COVID-19 patients indicate long-term elevated levels of acute phase proteins [14][19]. In turn, this may be a sign of an ongoing background systemic inflammatory process. As known, patients with MI and higher CRP levels have worse immediate and long-term prognosis compared with patients with low CRP [20].
A higher troponin I level in the experimental group may not only indicate the volume of ischemic myocardium, but also signs of previously described ongoing COVID-19-associated myocarditis [21]. A common finding in COVID-19 patients is an increase in high-sensitivity troponin level [22]. However, there are very few reliable data on the direct cytopathic effect of SARS-CoV-2 on cardiomyocytes [23]. In the observational study [24], according to cardiac magnetic resonance imaging, patients after COVID-19 showed higher end-diastolic volume, myocardial mass, and increased longitudinal and transverse relaxation times T1 and T2. Myocardial biopsy showed histological signs of inflammation and lymphocytic infiltration [24].
The presented results obtained in NSTEMI patients were compared with the data published earlier in largescale Russian registries RECORD 1-3, LIS 1,3, Moscow registry [25-28] and international registries Euro Heart Survey on acute coronary syndromes II (EHS-ACS-II), long-tErm follow uP of antithrombotic management patterns In acute CORonary syndrome patients (EPICOR) and Global Registry of Acute Coronary Events (GRACE) [29-31]. In terms of demographic and clinical characteristics, comorbidities, patients in the control group, which included those without prior COVID-19, were comparable with the register data, which, despite the relatively small sample size (n=209), generally indicates its representativeness.
The main study limitations are the small sample size, single-center design, the high incidence of asymptomatic and mild COVID-19 among patients with NSTEMI, which requires a separate and more detailed analysis. The revealed differences in NSTEMI patients who underwent COVID-19 require further study, correlation and factor analysis.
Conclusion
Patients with NSTEMI after COVID-19 were significantly younger compared to patients without a history of COVID-19. They had less common exertional angina prior to MI, while C-reactive protein and troponin I levels were significantly higher than in the control group. There were no significant differences in echocardiographic parameters with the control group, while the estimated PASP was significantly higher compared to patients without prior COVID-19. The average period for MI development after COVID-19 was 49 days [ 34,0; 82,0 ].
The data obtained make it possible to consider prior COVID-19 as a potential trigger for an earlier manifestation of coronary artery disease in the form of MI in patients who already had other cardiovascular risk factors. The early NSTEMI after COVID-19, which falls within the interval of “symptomatic COVID-19”, indicates a critically important selection of time periods after the acute phase of COVID-19, which are the most dangerous in terms of CVE risk. These data can make a significant contribution to the optimization of diagnostic and treatment measures aimed at effective prevention of CVE in post-COVID-19 period.
Further identification of CVE risk factors and their significance, as well as terms for developing complications after COVID-19 is currently of great interest and is a topic for future research.
Relationships and Activities: none.
References
1. COVID-19 Map — Johns Hopkins Coronavirus Resource Center n.d. https://coronavirus.jhu.edu/map.html. (accessed September 9, 2021).
2. Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54:12- 6. doi:10.1016/J.JMII.2020.05.001.
3. Lam MH-B, Wing Y-K, Yu MW-M, et al. Mental Morbidities and Chronic Fatigue in Severe Acute Respiratory Syndrome Survivors: Long-term Follow-up. Arch Intern Med. 2009;169:2142-7. doi:10.1001/ARCHINTERNMED.2009.384.
4. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi:10.1136/BMJ.M3026.
5. National Institute for Health and Care Excellence, Practitioners RC of G, Scotland HI. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE Guidel. 2020:1-35.
6. Wu J, Mamas M, Rashid M, et al. Patient response, treatments, and mortality for acute myocardial infarction during the COVID-19 pandemic. Eur Hear J — Qual Care Clin Outcomes. 2020;0:1-9. doi:10.1093/ehjqcco/qcaa062.
7. Chughtai AA, Tan TC, Hitchen EM, et al. Association of influenza infection and vaccination with cardiac biomarkers and left ventricular ejection fraction in patients with acute myocardial infarction. IJC Hear Vasc. 2020;31:1-6. doi:10.1016/j.ijcha.2020.100648.
8. Vejpongsa P, Kitkungvan D, Madjid M, et al. Outcomes of Acute Myocardial Infarction in Patients with Influenza and Other Viral Respiratory Infections. Am J Med. 2019;132:1173-81. doi:10.1016/j.amjmed.2019.05.002.
9. Kaminski KA, Kozuch M, Bonda T, et al. Coronary sinus concentrations of interleukin 6 and its soluble receptors are affected by reperfusion and may portend complications in patients with myocardial infarction. Atherosclerosis. 2009;206:581-7. doi:10.1016/j.atherosclerosis.2009.03.033.
10. Van Den Berg VJ, Umans VAWM, Brankovic M, et al. Stabilization patterns and variability of hs-CRP, NT-proBNP and ST2 during 1 year after acute coronary syndrome admission: Results of the BIOMArCS study. Clin Chem Lab Med. 2020;58:2099-106. doi:10.1515/cclm-2019-1320.
11. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-69. doi:10.1093/EURHEARTJ/EHY462.
12. United Nations. The Impact of COVID-19 on Women 2020. https://www.unwomen.org/-/media/headquarters/attachments/sections/library/publications/2020/policy-brief-the-impact-of-covid-19-on-women-en.pdf?la=en&vs=1406. (accessed October 13, 2021).
13. Liakos M, Parikh PB. Gender Disparities in Presentation, Management, and Outcomes of Acute Myocardial Infarction. Curr Cardiol Reports. 2018;20:1-9. doi:10.1007/S11886-018-1006-7.
14. Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57:1-11. doi:10.1183/13993003.03481-2020.
15. Syrov AV, Sturov NV, Kolupaev VE, et al. Diagnosing COVID-19 in Outpatient Practice. Tr Patsient. 2020;18(5):6-9. (In Russ.) doi:10.24411/2074-1995-2020-10031.
16. Popova AYu, Ezhlova EB, Mel’nikova AA, et al. Herd Immunity to SARS-CoV-2 among the Population in Saint-Petersburg during the COVID-19 Epidemic. Problems of Particularly Dangerous Infections. 2020;3:124-30. (In Russ.) doi:10.21055/0370-1069-2020-3-124-130.
17. Ing AJ, Cocks C, Green JP. COVID-19: in the footsteps of Ernest Shackleton. Thorax. 2020;75:693-4. doi:10.1136/THORAXJNL-2020-215091.
18. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25:1-6. doi:10.2807/1560-7917.ES.2020.25.10.2000180.
19. Chashchin MG, Gorshkov AYu, Drapkina OM. Acute coronary syndrome in COVID-19 patients. Cardiovascular Therapy and Prevention. 2021;20(5):2806. (In Russ.) doi:10.4103/1995-705X.96660.
20. Sheikh AS, Yahya S, Sheikh NS, et al. C-reactive Protein as a Predictor of Adverse outcome in Patients with Acute Coronary Syndrome. Heart Views. 2012;13:7. doi:10.4103/1995-705X.96660.
21. Li C, Jiang J, Wang F, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74- 87. doi:10.1016/j.yjmcc.2020.08.008.
22. Chen C, Yan JT, Zhou N, et al. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:567-71. doi:10.3760/cma.j.cn112148-20200225-00123.
23. Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097-100. doi:10.1093/cvr/cvaa078.
24. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265-73. doi:10.1001/JAMACARDIO.2020.3557.
25. Erlikh AD Twelve months outcomes in patients with acute coronary syndrome, by the national registry RECORD-3. Russ J Cardiol. 2018;(3):23-30. (In Russ.) doi:10.15829/1560-4071-2018-3-23-30.
26. Marcevich SYu, Ginzburg ML, Kutishenko NP, et al. The LIS study (lyubertsy study of mortality in patients with acute myocardial infarction). Evaluation of the pharmacotherapy. Part 1. Treatment of patients before myocardial infarction and its influence on hospital mortality rate. Rational Pharmacotherapy in Cardiology. 2012;8(5):681-4. (In Russ.) doi:10.20996/1819-6446-2012-8-5-681-684.
27. Martsevich SYu, Semenova YuV, Kutishenko NP, et al. LIS-3 register of the acute coronary syndrome: What has changed in a “portrait” of a patient and short-term outcomes of the disease compared to LIS-1 register. Rational Pharmacotherapy in Cardioljgy. 2017;13(1):63-8. (In Russ.) doi:10.20996/1819-6446-2017-13-1-63-68.
28. Erlikh AD The First Moscow Acute Coronary Syndrome Registry: the results of six-month follow-up. Emergency Cardiology. 2014;2:3-9. (In Russ.) Э
29. Mandelzweig L, Battler A, Boyko V, et al. The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J. 2006;27:2285-93. doi:10.1093/EURHEARTJ/EHL196.
30. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153:29-35. doi:10.1016/J.AHJ.2006.10.004.
31. Pocock S, Bueno H, Licour M, et al. Predictors of one-year mortality at hospital discharge after acute coronary syndromes: A new risk score from the EPICOR (long-tErm follow uP of antithrombotic management patterns In acute CORonary syndrome patients) study. Eur Heart J. Acute Cardiovasc Care. 2015;4:509-17. doi:10.1177/2048872614554198.
About the Authors
M. G. ChashchinRussian Federation
Moscow
A. Yu. Gorshkov
Russian Federation
Moscow
O. M. Drapkina
Russian Federation
Moscow
I. V. Kositsyna
Russian Federation
Moscow
A. V. Golubev
Russian Federation
Moscow
N. I. Chaus
Russian Federation
Moscow
S. N. Perekhodov
Russian Federation
Moscow
Supplementary files
Review
For citations:
Chashchin M.G., Gorshkov A.Yu., Drapkina O.M., Kositsyna I.V., Golubev A.V., Chaus N.I., Perekhodov S.N. Clinical and anamnestic characteristics of patients with non-ST elevation myocardial infarction after COVID-19. Cardiovascular Therapy and Prevention. 2021;20(7):3062. https://doi.org/10.15829/1728-8800-2021-3062
JATS XML

























































