Scroll to:
Impact of acute and chronic regular exercise on arterial stiffness and reflection measures in coronary artery disease patients: A Protocol for Randomized Clinical Trial
https://doi.org/10.15829/1728-8800-2022-3362
Abstract
Vascular stiffness due to arteriosclerosis reduces arterial elasticity which is as an independent and non-invasive predictor of future incidence of coronary heart disease and stroke. There is limited evidence of the benefits of regular exercise on arterial stiffness in populations suffering from cardiovascular diseases (CVD) especially coronary artery disease (CAD).
Aim. To determine the acute and chronic effects of aerobic, resistance and combined exercise on arterial and hemodynamic function in patients with CAD.
Material and methods. This study will be a prospective, single-blind, randomized study examining the effects of exercise on arterial stiffness. The study will be conducted at a tertiary care hospital for a continuous period of one year. Patients with CAD (n=105) will be selected using systematic sampling techniques and allocated randomly to one of the four treatment groups using computer-generated, random number sequence for age, sex and health status of CAD (Group-I: aerobic exercise, Group-II: resistance exercise, Group-III: combined aerobic and resistance exercise, and Group-IV: control) as per the inclusion and exclusion criteria. All recruited subjects will be informed about the assessment and intervention procedure before getting the consent form filled. This project followed the guidelines of standard protocol for randomized clinical trials (spirit).
Perspective. It is expected that this study protocol shall through light and be helpful in better quality of life along with decline in drug dependency for the people suffering from CAD. Further this study will be valuable is designing exercise protocol for the people suffering from CAD.
Keywords
For citations:
Kapoor G., Swaroop A., Singh S. Impact of acute and chronic regular exercise on arterial stiffness and reflection measures in coronary artery disease patients: A Protocol for Randomized Clinical Trial. Cardiovascular Therapy and Prevention. 2022;21(10):3362. https://doi.org/10.15829/1728-8800-2022-3362
Introduction
By 2030 cardiovascular diseases (CVD) are predicted to be a leading cause of death worldwide, with measures for early detection [1] and progress monitoring essential to manage the condition and its associated health costs. Coronary Heart Disease (CHD) has been ranked at first position in causing mortality, followed by pulmonary disorders and cancer respectively [2]. In the developed countries significant progress has already been made in fighting against CHD, but the death rate following CVD is continuously increasing in developing countries since last 50 years [3]. In India, studies reported soaring in CVD, consecutively in the last 60 years from 1 to 9-10% in urban areas and <1 to 4-6% in rural locations [4]. Vascular stiffness due to arteriosclerosis reduces arterial elasticity which is as an independent non- invasive predictor of future incidence of CHD, stroke and mortality [5]. Further, greater vascular stiffness has been considered as one of the leading signs of vascular damage leading to CVD [6]. Vascular stiffness changes linearly with age and augmented arterial stiffness has been associated with long-term, poor lifestyle factors like inadequate diet, lack of physical activity, and smoking [7]. Altered vascular stiffness is implicated as leading hemodynamic element inf luencing Augmentation Index (AIx); a measure of wave ref lection and left ventricular after load, and sensitive marker of future CVD risk particularly in younger individuals [8]. Central Pulse Wave Velocity (PWV) is indicative of stiffness of the large elastic arteries such as the aorta (descending and ascending) and carotid artery. Central PWV is used for predicting of CVD as the central aorta is the most affected by conventional risk factors like age, sex, smoking, diabetes mellitus, dyslipidemia or elevated cholesterol levels and arterial hypertension [9]. Elevated central PWV is associated with increased Systolic Blood Pressure (SBP) symbolize a greater load on the left ventricle and reduced coronary perfusion [10].
Exercise-based cardiac rehabilitation (aerobic endurance training, dynamic resistance and stretching training) have been used to manage cardiovascular health in individuals with CVD. Regular exercise has been associated with a 35% reduction in CVD mortality and 33% reduction in all-cause mortality when compared to sedentary individuals [11]. However, depending on the exercise, conf licting results have been found regarding its effect on arterial stiffness. Aerobic exercise is well accepted to reduce CVD risk and is associated with reduced large-artery stiffness [12][13]. In contrast, resistance training, a recommended form of exercise to improve muscular strength, has been shown to induce negative effects, both acutely and chronically on arterial stiffness [14]. When combined, both aerobic and resistance exercise training have been shown to have a small or negligible positive effect on arterial stiffness [15].
Further, a meta-analysis revealed aerobic training to result in significantly lower PWV than combined training [16][17]. The same meta-analysis along with other meta-analysis also have indicated combined aerobic and resistance training while yielding a non-significant decrease in PWV has less impact on arterial stiffness than aerobic training alone [16][17]. A study proved PWV to decrease immediately following aerobic exercise in both normotensive and hypertensive individuals [18]. The same study supported acute endurance exercise leads not only to decreased blood pressure but even reduces aPWV as a measure of arterial stiffness even after 60 min of recovery [19]. Moreover, the same study proved that relatively short aerobic exercise intervention in older adults can reduce PWV in multifactorial arterial stiffness [12].
Hence, we may predict that exercise have been examined extensively in healthy populations and those with arterial hypertension. Moreover, regular exercise shows beneficial effect on CAD patients. However, there is limited evidence of the benefits of regular exercise on arterial stiffness in populations suffering from CVD, especially CAD. The planned study aims to determine the acute and chronic effects of aerobic, resistance and combined exercise on arterial hemodynamic function (i.e. PWV, AIx) in patients with CAD.
Material and methods
Trial registration. The study protocol has been approved by the Institutional Ethics Committee, Jayoti Vidyapeeth Women’s University, Jaipur on April 10, 2021. This proposed trial has been registered with ClinicalTrials.gov ID: CTRI//2021/10/037127. The study would be conducted according to National Ethical Guidelines for Biomedical Research involving Human participants, 2017 and Helsinki Declaration revised in 2013.
Study design. This study will be a prospective, singleblind, randomized study examining the effects of exercise on arterial stiffness. The study will be conducted at a tertiary care hospital for a period of one year from October, 2021 to September, 2022. Patients will be selected using systematic sampling techniques having CAD (n=84; 21 per group) will be randomized to one of four treatment groups (Group-I: aerobic exercise, Group-II: resistance exercise, Group-III: combined aerobic and resistance exercise, and Group-IV: control group, see Figure 1). Subjects of Groups I, II and III will undergo 8 weeks of supervised training, three times per week in accordance with recommended guidelines from American College of Sports Medicine (ACSM).
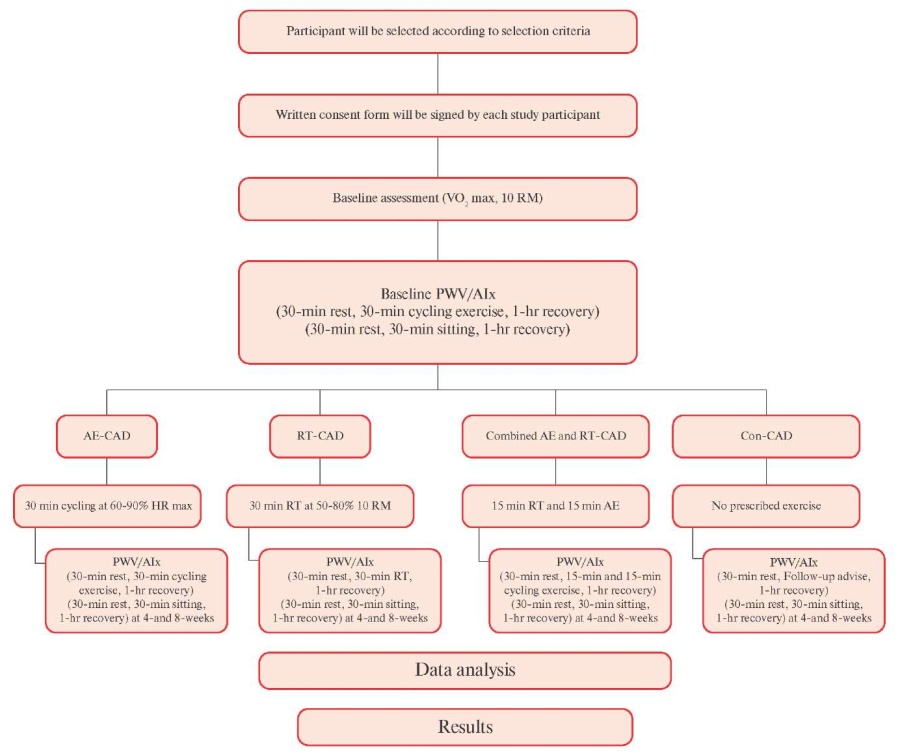
Figure 1 Flow Chart for Study Protocol.
Participant Recruitment. 105 subjects with CAD from the tertiary care hospital will be recruited using systematic sampling techniques. Recruitment of subjects will begin in the first week of October 2021 and finished in September 2022. All subjects will be informed about the assessment and intervention procedure before getting the consent form filled. Confidentiality of patient and collected data will be maintained.
Inclusion criteria. Presence of CAD, which is defined as the patient having at least one of the following: angiographically documented stenosis ≥50% in at least one major coronary artery; prior history of Myocardial Infarction (MI), Percutaneous Coronary Intervention (PCI), or Coronary Artery Bypass Graft (CABG) surgery; positive exercise stress test determined by a positive nuclear scan, or symptoms of chest discomfort accompanied by Electro Cardio Graphic (ECG) changes of >1 mm horizontal or down sloping ST segment depression.
Exclusion criteria. Non-cardiac surgical procedure within two months, MI or CABG within two months, PCI within one month, NYHA class II-IV symptoms of heart failure, documented valve stenosis, documented severe chronic obstructive pulmonary disease, symptomatic peripheral arterial disease, unstable angina, uncontrolled hypertension, uncontrolled atrial arrhythmia or ventricular dysrhythmia, insulin requiring diabetes mellitus, and any musculoskeletal abnormality that would limit exercise participation.
Outcomes
Primary Outcomes
The primary outcome will be measurement of PWV and AIx. PWV and AIx will be measured non-invasively using color Doppler ultrasound. The evaluations will be carried out at baseline, at end of 4th and 8th weeks for individuals in all the groups.
Pulse Wave Velocity (PWV)
Assessment of arterial stiffness by carotid-femoral PWV measurement. Carotid-femoral PWV (cf-PWV) is the velocity of the arterial pulse wave between the carotid and femoral arterial sites [20], which is commonly assessed using applanation tonometry. Cf-PWV is highly reliable and valid in predicting the severity of the CAD with positive correlation coefficient between cf-PWV and CAD (r=0.838, p=0.001) [21].
For measuring the PWV the study will use the SphygmoCor (AtCor Medical, Sydney, Australia. Model MM3. Software version 7.01 S) with one tonometric Millar transducer. SphygmoCor helps to measure the cf-PWV in following two steps. In the first step it will record carotid pulse wave along with ECG, and during second step femoral pulse wave and ECG will be recorded. It is recommended to do ECG recording as it is essential in synchronization of carotid and femoral pulse wave times. Transit time between carotid and femoral pressure waves will be measured using the foot-tofoot method. Intersecting tangent algorithms are used to find the ‘foots’ of waves. PWV is measured using this method by measuring the pulse transit time and distance traversed by the pulse wave. With SphygmoCor the most common approach entails measuring two distances on the body surface, namely from the sternal notch to the femoral site and from the sternal notch to the carotid location of corresponding pulse wave recordings. The travelled distance is determined automatically when data is entered into the computer as the difference between the two distances, femoral location-sternal notch minus sternal notch-carotid location. This approach will be employed to compute travelled distance in this study, as most of the studies used this method for measuring cf-PWV using SphygmoCor [22].
Augmentation index (AIx)
AIx will be assessed as the augmentation in the pressure from the initial shoulder in the ascending trace of aortic waveform to the peak of the wave (Δ P), expressed as a percentage of the pulse pressure [23].
AIx (in percentage)=(central P2-P1 [augmented pressure]/central pulse pressure)×100. The time (in millisec.) from the start of the systolic upstroke to the first inflection point was used to compute the timing of the returning reflected wave, which serves as a marker for aortic PWV (stiffness). SphygmoCor software includes an algorithm that normalises AIx to a heart rate of 75 bpm (based on literature). AIx is highly reliable and valid in predicting the progress of CAD [24] (Figure 2).
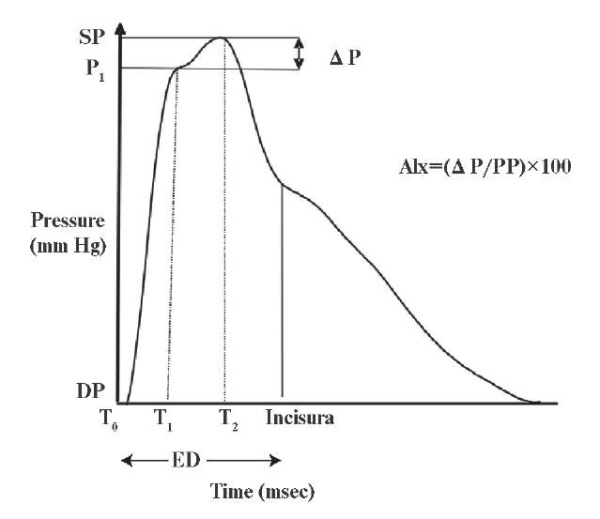
Figure 2 Augmented Index measurement [23].
Note: T0 — time at the start of the waveform, T1 — duration from start of waveform to the first peak/shoulder (outgoing pressure wave), T2 — duration from start of waveform to the second peak/shoulder (reflected pressure wave), ED — ejection duration, or duration from start of waveform to closure of the aortic valve (incisura), SP — central aortic systolic pressure, DP — central aortic diastolic pressure, P1, P1 height difference between the minimum pressure and the pressure at the first peak/shoulder (T1), augmentation — Δ P, difference between maximal pressure (central aortic systolic pressure) and pressure at the fist peak/ shoulder (P1 height), PP — pulse pressure, and AIx — augmentation index.
Secondary Outcomes
The secondary outcome will be measurement of SBP, diastolic blood pressure and HR will be measured using calibrated automatic digital blood pressure monitor.
Randomization. Prior to randomization, all participants will complete a screening and familiarisation session including the assessment of VO2 max, cycling exercise and10-RM (bench press, leg press, biceps curl, etc). All participants will be allocated in one of the four groups according to a computergenerated sequence number with randomization stratified for age, sex and health status of CAD (e.g. vessel involvement). During the 1st visit or on the day of baseline assessment subjects will be instructed to take rest for 30 min. followed by familiarisation session of aerobic training, resistance training, combined training and advise for follow-up (according to the group assigned) for 30 min. with 1 hour recovery phase.
Baseline measurements will be recorded after one hour of recovery period following familiarization session: 1) HR and blood pressure, 2) assessment of arterial stiffness by cf-PWV measurement, 3) AIx, a measure of wave reflection and left ventricular after load, and diagnostically useful indicator of future CVD risk. Participants will be instructed to sit for 30-60 min. after the evaluation before returning home.
Interventions. Each training session will include 30 min. rest period before the initiation of the training phase, 10 min. of standardized warm-up and cool-down involving light aerobic exercise and upper and lower body stretching, 30 min. training phase and 1 hour recovery period.
Group-I (aerobic training) will consist of cycling between 25-30 min. at an intensity of 60-90% percent of maximal HR [25][26]. Group-II (resistance training) will involve upper and lower body exercises (3 sets of 8-12 repetitions) at an intensity of 50-80% of 10 repetition maximum (RM, ~30 min) [27].
Group-III (combined training) will involve a combination of aerobic and resistance training exercises, as described above for an equivalent time (25-30 min. consisting of 12-15 min. of AE and RT each) [28].
During all sessions, participants will be instructed to fast for at least 8 hours, and to abstain from exercise for 24 hours, and caffeine and alcohol consumption for 12 hours. Medications and vitamins will be kept constant throughout the study, except for nitroglycerin, which will be withheld on the days of intervention. Height in cm and weight in kg will be measured, and Body Mass Index (BMI) will be calculated.
During the 8-week exercise period, measurements of PWV and AIx will be repeated at end of 4th weeks and 8th weeks for individuals in all the groups after 30 min. of rest period. Following measurement participants will be made to relax for 30 min. in sitting position for recovering from investigation stress. All participants will give consent for their volunteer participation in the study, which is approved by the Institutional Ethics Committee (Figure 3).
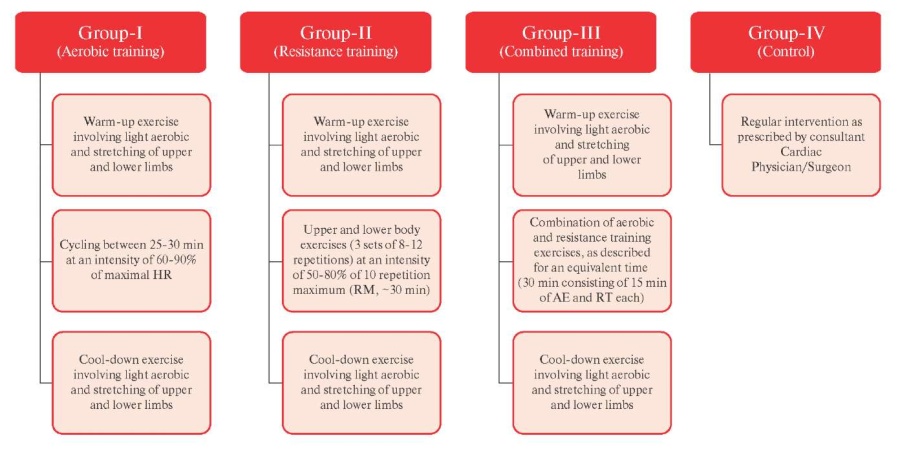
Figure 3 Flow chart of Intervention.
Note: AE — Aerobic exercise, HR — heart rate, RM — Repetition maximum, RT — Resistance training.
Blinding. It is non-viable to blind the interventional team given the nature of the interventions in single-blind project, as the researcher and therapist will incorporate the exercise program in all the allocated groups. Further third person will perform both the randomization and the blind allocation for the study participants along with replacing the participant’s name with the allocation number in the analysis software. However, the type of intervention will be concealed from the primary outcome assessor staff. In addition, best possible effort will be used to blind the interventional subjects. First, they will not be informed of their group assignment. Second, interventions will be offered to each of the four groups in a different room. Third, the initial assessment of the subjects will be done on different days and times so that they will neither recognize with each other nor aware about the allocated interventional group. Fourth, type of exercise during warm-up and cool-down is of similar nature. This procedure will helps in blinding of the study subjects.
Safety Control. Intensity and time period of exercise will be increased gradually as the subject feel assertive with sufficient amount of warm-up and cool-down period. Further, subjects will be advised to take medications as advised. Subjects will be further advised to take plenty of water before, during and after the exercise session to remain hydrated. Subjects will be advised to wear loose clothes, well-fitted shoes and socks. All the subjects will be monitored for 30 min. after the exercise session as preventive measures. Subjects will be asked to report and stop the exercise if feel symptoms of chest pain, breathlessness, dyspnea, cramps, calf muscle pain, light headedness, target HR higher than age, abrupt rise or fall in the BP. In case of causality emergency medications and healthcare team will be available on call.
Adverse Events. Researchers shall assess vital signs in the beginning, during and after completion of each session in group I, II and III. In case of symptoms of chest pain, breathlessness, dyspnea, cramps, calf muscle pain, light headedness, the session shall be stopped for the particular participant. Any recurrent occurring adverse event threatening to life or related to significant disability will be reported to the Ethics Research Committee of the Institution.
Data Monitoring. The researcher will perform the statistical analyses and datasets. Therapist shall monitor the treatment sessions of all designed groups.
Statistical issues. The sample size has been calculated using the G*power tool. Sample size calculated using Gpower 3.1 software and effect size of 0.0862439 found to be 84 (21 for each group) [29]. Considering the dropout rate of 20%, 105 subjects will be recruited for the present study. For reducing the dropout rate study will give flexibility in timing for the follow-up with listening to subjects problems and boosting up the confidence level of the study participants, during the day of visit. Reminder phone calls and e-mails will be sent to the study participants for follow-up. Junior physiotherapist under the supervision of researcher will do the follow-up with the study participants.
Data Analysis. The data collected will be analyzed by the same researcher. Descriptive statistics will be used to evaluate the baseline characteristics of the participating subjects. The normality of the collected data will be established using Kolmogorov-Smirnov test. Based on data normality, descriptive statistics will be expressed as mean±standard deviation or median and intra-quartile range.
For acute effect of exercise for four weeks, study will analyze results using Paired t-test and Wilcoxon signed rank test within group and independent t-test and Mann-Whitney U test between the four groups if the data is normally or non-normally distributed, respectively. Further for analyzing the chronic effect of exercise statistical test repeated measure ANOVA and Friedman test within group and one way ANOVA and Kruskal-Wallis test between four groups will be used if the data is normally or non-normally distributed, respectively (Table 1). For intergroup analysis of the data Bonferroni correction method will be used to minimise the type-I error. Data will be analyzed using the statistical software (SPSS v 26.0. SPSS Inc. Chicago, IL, USA.). Level of significance will be set at 0.05.
Table 1
Test used for data analysis

Data Management. The collected data shall be kept confidential with the primary researcher throughout the duration of the study and discarded post 5 years from commencement of the study. Initial documentation of data will be done through paper printed data collection forms which will later be managed and transcribed into electronic format of SPSS v 26.0 and stored in a desktop without an internet connection to help prevent unauthorized data access for further analysis.
Perspective
It is expected that this study protocol should shed some light and be helpful in better quality of life along with decline in drug dependency. Further this study will be valuable is designing exercise protocol for the people suffering from CAD.
Final Considerations
To date, there is no study which has documented the different forms exercise training on PWV and AIx among CAD patients. The authors of this study have carefully selected the research design to control the progress of the disease with non-pharmacological interventions.
Strength and Limitation
The planned study will be a single blinded. Researchers will be well aware of the interventions provided to the three groups. This will be perceived as a potential source of unbiasing in data collection. Further, due to the quantitative nature of the data collected, measurement bias is likely to be there. The researchers will make all efforts for the success of this study making use of interpersonal skills of the researcher, establish a great rapport with the participants.
As this a single site regional study, we will not able to implement the findings for global population. Future studies with multi-centric site within the country and/ or Globe can be possible with the reference of this single centre study.
As this is a single site short duration study finding the effect physical exercise contributor on arterial stiffness will be studied, further studies can correlate psychological changes with exercise for changes in arterial stiffness.
A short duration with small sample size study can help to address the research question in short interval of time. As this will be new intervention study in CAD, will helps to control numerous resources.
Relationships and Activities: none.
References
1. Mc Namara K, Alzubaidi H, Jackson JK. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved? Integr Pharm Res Pract 2019;8:1-11. doi:10.2147/IPRP.S133088.
2. Arsenault BJ, Rana JS, Stroes ES, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-highdensity lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. 2009;55(1):35-41. doi:10.1016/j.jacc.2009.07.057.
3. Yusuf S, Reddy S, Ôunpuu S, et al. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746-53. doi:10.1161/hc4601.099487.
4. Nag T, Ghosh A. Cardiovascular disease risk factors in Asian Indian population: A systematic review. J Cardiovasc Dis Res. 2013;4(4):222-8. doi:10.1016/j.jcdr.2014.01.004.
5. Duprez DA, Cohn JN. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. 2007;9(2):139-44. doi:10.1007/s11883-007-0010-y.
6. Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res 2016;113(Pt A):600-09. doi:10.1016/j.phrs.2016.09.040.
7. DuPont JJ, Kenney RM, Patel AR, et al. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol 2019;176(21): 4208-4225. doi:10.1111/bph.14624.
8. McEniery CM, Yasmin, Hall IR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46(9):1753-60. doi:10.1016/j.jacc.2005.07.037.
9. Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636-46. doi:10.1016/j.jacc.2013.09.063.
10. Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17(4-5):545-54. doi:10.1007/s10741-011-9270-2.
11. Nocon M, Hiemann T, Müller-Riemenschneider F, et al. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239-46. doi:10.1097/HJR.0b013e3282f55e09.
12. Madden KM, Lockhart C, Cuff D, et al. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32(8):1531-5. doi:10.2337/dc09-0149.
13. Donley DA, Fournier SB, Reger BL, et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol 2014;116(11):1396-04. doi:10.1152/japplphysiol.00151.2014.
14. García-Mateo P, García-de-Alcaraz A, Rodríguez-Peréz MA, et al. Effects of resistance training on arterial stiffness in healthy people: A systematic review. J Sports Sci Med. 2020;19(3):444. doi:10.1136/bjsm.2008.052126.
15. Park W, Jung WS, Hong K, et al. Effects of moderate combined resistance-and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int J Environ Res Public Health. 2020;17(19):7233. doi:10.3390/ijerph17197233.
16. Ashor AW, Lara J, Siervo M, et al. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(10):e110034. doi:10.1371/journal.pone.0110034.
17. Montero D, Vinet A, Roberts CK. Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol. 2015;178:69-76. doi:10.1016/j.ijcard.2014.10.147.
18. Seo JB, Chung WY, Kim HS, et al. Immediate impact of exercise on arterial stiffness in humans. World J Cardiovasc Dis. 2013;3(1):40-5. doi:10.4236/wjcd.2013.31009.
19. Milatz F, Ketelhut S, Ketelhut RG. Favorable effect of aerobic exercise on arterial pressure and aortic pulse wave velocity during stress testing. Vasa. 2015;44(4):271-6. doi:10.1024/0301-1526/a000441.
20. Pereira T, Correia C, Cardoso J. Novel Methods for Pulse Wave Velocity Measurement. J Med Biol Eng. 2015;35(5):555-65. doi:10.1007/s40846-015-0086-8.
21. Kim HL, Kim SH. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc Med. 2019;6:41. doi:10.3389/fcvm.2019.00041.
22. Rajzer MW, Wojciechowska W, Klocek M, et al. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens. 2008; 26(10):2001-7. doi:10.1097/HJH.0b013e32830a4a25.
23. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213-25. doi:10.1161/CIRCULATIONAHA.105.595496.
24. Sharma KH, Sharma N, Shah K, et al. Impact of coronary artery disease on augmentation index as measured by estimated central blood pressure: A case control study in Asian Indians. Indian Heart J. 2018;70(5):615-21. doi:10.1016/j.ihj.2017.12.001.
25. Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise Standards for Testing and Training: A Statement for Healthcare Professionals From the American Heart Association. Circulation. 2001;104(14):1694-740. doi:10.1161/hc3901.095960.
26. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-59. doi:10.1249/MSS.0b013e318213fefb.
27. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687-708. doi:10.1249/MSS.0b013e3181915670.
28. Ho SS, Dhaliwal SS, Hills AP, et al. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. doi:10.1186/1471-2458-12-704.
29. Moon SH, Moon JC, Heo DH, et al. Increased pulse wave velocity and augmentation index after isometric handgrip exercise in patients with coronary artery disease. Clin Hypertens. 2015;21:5. doi:10.1186/s40885-015-0016-7.
About the Authors
G. KapoorIndia
Gaurav Kapoor - PhD Scholar, Department of Physiotherapy.
Jaipur, Rajasthan, +91987-240-07-11
A. Swaroop
India
Avinash Swaroop - Professor.
Jaipur, Rajasthan
S. Singh
India
Sandeep Singh - Assistant professor, Department of Physiotherapy.
Patiala, Punjab
Supplementary files
Review
For citations:
Kapoor G., Swaroop A., Singh S. Impact of acute and chronic regular exercise on arterial stiffness and reflection measures in coronary artery disease patients: A Protocol for Randomized Clinical Trial. Cardiovascular Therapy and Prevention. 2022;21(10):3362. https://doi.org/10.15829/1728-8800-2022-3362
JATS XML

























































