Scroll to:
Positive effects of renal denervation on markers of cardiovascular inflammation and left ventricular mass. 24-months follow-up
https://doi.org/10.15829/1728-8800-2021-2678
Abstract
Aim. To study the long-term effect of renal denervation (RDN) on left ventricular mass (LVM) and inflammatory markers in resistant hypertensive patients.
Material and methods. Forty-one patients with resistant hypertension and 24-h blood pressure (BP) 158,7±15,8/87,3+14,6 mmHg, aged 56,6+10,2 years, were enrolled in the study and undergone RDN. Mean 24-h BP, left ventricular mass (transthoracic echocardiography), high sensitivity C-reactive protein (hsCRP), interleukin- 1β (IL1β), IL-6, IL-10) and tumor necrosis factor alpha (TNF- α) were assessed at baseline and 2 years after the RDN.
Results. A baseline prevalence of left ventricular hypertrophy (LVH) was 90,2%. Two years after RDN LVM and interventricular septum (IVS) decreased significantly (p<0.05 for both). Decrease in myocardial mass (∆LVM >0 g) was documented in 24 patients. The regression of LVM was accompanied by a significant decrease in levels of inflammatory markers — hsCRP by 38,3% (p=0,031), TNF-α by 60,7% (p=0,009), IL- 1β — by 71,1% (p=0,001), and IL-10 by 58,2% (p=0,001). In patients in the absence of LVM regression only TNF-α decreased significantly (-68,8%, p=0,001). There was no correlation between changes of LVM and the inflammatory markers at 24 months after RDN.
Conclusion. The RDN in RH patients may have long-term cardioprotective effect in terms of significant regress of LVH, which may be partly attributed to the regress in systemic or myocardial inflammation.
Keywords
For citations:
Sitkova E.S., Mordovin V.F., Pekarskiy S.E., Ripp T.M., Ryabova T.R., Falkovskaya А.Yu., Lichikaki V.A., Zyubanova I.V., Baev A.Е., Gusakova A.M. Positive effects of renal denervation on markers of cardiovascular inflammation and left ventricular mass. 24-months follow-up. Cardiovascular Therapy and Prevention. 2021;20(2):2678. https://doi.org/10.15829/1728-8800-2021-2678
Introduction
Despite the progress in the development of antihypertensive pharmacotherapy a prevalence of uncontrolled arterial hypertension (AH) is still very high. Major classes of antihypertensive drugs can reduce blood pressure (BP), on average, by -9.1/-5.5 mm Hg when administered as monotherapy and around -30/ -15 mm Hg when 3 drugs are used in combination [1]. It is obviously not enough for effective treatment of severe AH with BP higher than 180 mm Hg. Therefore, a significant proportion of treated hypertensives remain uncontrolled or treatment-resistant. The risk of stroke and myocardial infarction in patients with resistant hypertension (RH) is twice as high as in those with controlled hypertension.
According to meta-analysis of cross-sectional and longitudinal studies including 3325 patients, left ventricular hypertrophy (LVH) is diagnosed in 55-75% of patients with RH [2]. A negative effect of LVH on clinical course and the outcomes of myocardial infarction has been shown in a large observational study [3]. Also, LVH negatively affected the infarct size in experimental research [4]. The LVH is independent predictor of systolic dysfunction [5], sudden cardiac death and cardiovascular death. Reduction of LVH or left atrium size due to antihypertensive pharmacotherapy can have a positive effect on a risk of major cardiovascular events [6].
A role of inf lammation in the development of AH and hypertensive organ damage has been widely discussed in recent decades. A variety of proinflammatory and inflammatory markers have been studied for their relationship with the progression of the disease, especially, high sensitivity C-reactive protein (hsCRP), interleukins and tumor necrosis factors.
One of the most promising therapeutic developments potentially capable to solve the problem of RH is catheter based renal denervation (RDN). Treatment of RH with RDN demonstrates regress of LVH, but with low predictability, and even in the absence of BP response. Also, long-term effects of RDN on LVH are not well studied.
Aim of the study is to determine the long-term effect of RDN on left ventricular mass (LVM) and inf lammatory markers in patients with resistant hypertension.
Material and methods
We performed a retrospective analysis of the data from the single center study of RDN in RH patients conducted in the Cardiology Research Institute of Tomsk National Medical Research Center.
Study inclusion criteria were: hypertensive patients of both genders, 18-80 years old, office BP >160/90 mmHg (systolic /diastolic, respectively) despite treatment with maximum tolerated doses of 3 antihypertensive drugs in, one of which was a diuretic. All patients provided written informed consent before inclusion in the study. The patients were excluded if they had secondary hypertension, 24-h mean systolic blood pressure (SBP) <135 mm Hg, glomerular filtration rate (by MDRD formula) (eGFR) <30 mL/min/m2, pregnancy, extended disease of the renal arteries, severe comorbidity significantly increasing risk of the intervention.
The patients meeting eligibility criteria underwent RDN procedure and were followed up for a period up to 3 years. Patients were instructed to maintain concomitant drug therapy as stable as possible during the follow-up.
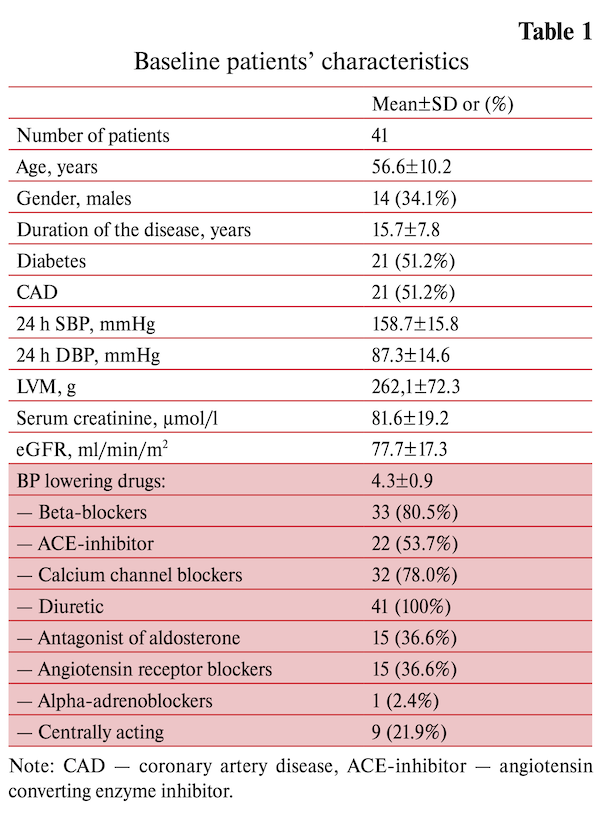
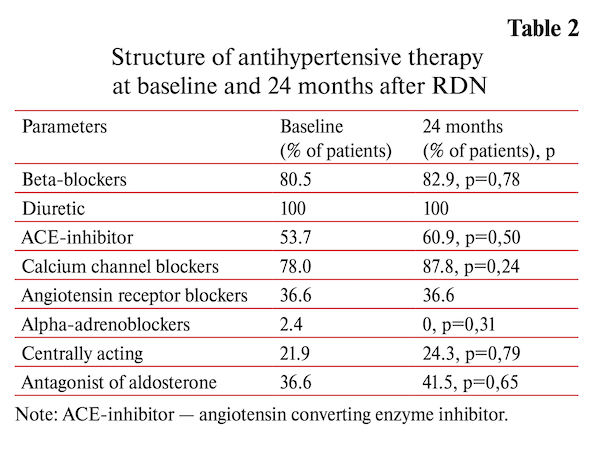
There were 6-, 12-, 24- and 36-months follow-up examination to assess the effectiveness and safety of renal denervation. The results of the annual observation were presented earlier. This study presents research data from 41 RH patients completed 2 year follow up. Baseline characteristics of the patients and structure of antihypertensive pharmacotherapy are summarized in the table 1. There were no deviation from the schedule of taking the drugs. Control was carried out by interviewing patients.
The following parameters were analyzed at baseline and 24 months after treatment:
- 24-h mean SBP and DBP obtained from ambulatory BP monitoring. Only data with more than 80% of successful measurements were accepted for the analysis.
- The thickness of interventricular septum (IVS) and posterior wall of left ventricle, LVM and indexed LVM assessed by transthoracic echocardiography. LVH was diagnosed if indexed LVM was more than 95 g/m2 and 115 g/m2 for women and men respectively.
- hsCRP, interleukin-1β (IL-1β), IL-6, IL-10 and tumor necrosis factor alpha (TNF-α) measured in a blood serum by enzyme-linked immunosorbent assay (ELISA) in the absence of acute inflammation or exacerbation of chronic pathology.
- Serum creatinine, eGFR (MDRD).
Statistical analysis: Measurement data that followed a normal distribution were expressed as mean±SD. Betweengroup differences in continuous (interval) variables were assessed using T-test, Chi-square test was used to asses differences in categorical variables. Continuous relationships between interval variables were evaluated using Pearson correlation coefficients, t statistic was used to assess a significance of the relationships. A p-value <0.05 was considered as significant.
Results
Two years after RDN the 24-hour BP was significantly decreased compared to baseline (-13.1/-7.4 mm Hg SBP/DBP respectively, p<0.001 for both). The analysis of patient-reported data on the antihypertensive drug use has shown no significant changes in concomitant drug therapy throughout the follow-up (4.14±0.89 vs 4.37±0.93, p<0.16) (table 2).
The baseline prevalence of LVH in the study sample was 90.2%. Two years after RDN LVM was decreased significantly from 269.9±71.7 to 254.6±58.1 g, р=0,048 mainly due to decrease in IVS from 14.0±1.5 to 13.5±1.5 g, р=0,015 whereas PW thickness did not change (12.9±1.7 g at baseline and 12.8±1.7 g, р=0,56).
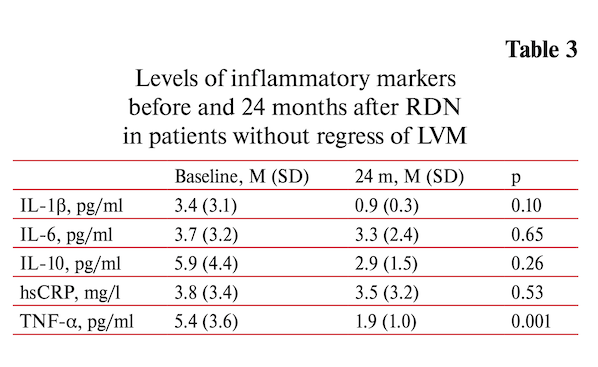
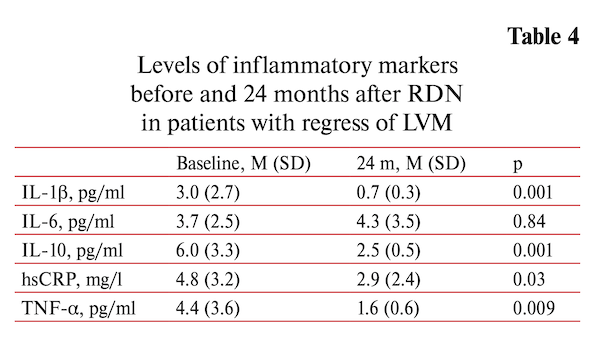
Decrease in myocardial mass (∆LVM >0 g) was documented in 24 patients. The regression of LVM was accompanied by significant decrease of inflammatory markers: the hsCRP by 38.3% (p=0.031), TNF-α by 60.7% (p=0.009), IL-1β — by 71.1% (p=0.001), IL-10 by 58.2% (p=0.001) except IL-6 that did not change (table 3).
In contrast, there was no significant changes inflammatory markers level in the absence of LVM regress (∆LVM <0 g, n=17). Only TNF-α decreased significantly (-68.8%, p=0.001) (table 4). In a both cases (with and without regress of LVH) BP decreased significantly at two years after RDN 24-h (-8.5/-5.8 and -17.3/-8.5 mm Hg respectively (p<0.01 for both).
There was no correlation between changes of LVM and inflammatory markers at 24 months after RDN. There were no serious adverse events associated with the RDN.
Discussion
RDN as a method of endovascular treatment of RH demonstrates cardioprotective effects but the magnitude of the effects is highly variable. Previous studies of cardioprotective efficacy of RDN were conducted in small patient groups and had a short duration, mainly, up to one year. The regression of LVM and atrial size after RDN was confirmed in metanalysis of 12 studies with twelve months follow up including 382 patients in total [7]. There was no relationship between cardiac changes and BP reduction after RDN in these studies. Regress of LVM and reduction of the volume of subendocardial damage assessed by contrast enhanced MRI were demonstrated in 35 RH patients over 1 year follow-up after RDN in our center [8]. Currently there are only a few publications on the longterm cardioprotective efficacy of RDN. The study in 18 patients with RH has shown a definite 24-month cardioprotective effect of RDN: regression of LVH was detected in 70.6% cases, the prevalence of concentric remodeling dropped by 47.1%. Cardiac changes were not related to the BP lowering after RDN [9].
The relationship between inf lammation and hypertension has been shown in a number of studies [10]. Levels of IL-1β, IL-6, TNF-α in hypertensive patients are significantly higher compared to normotensives [11]. In particular, it has been shown that hsCRP known as a marker of vascular inflammation and remodeling is also involved in the development of LVH [12], has strong association with hypertension [13] and predicts cardiovascular complications in hypertensive patients.
The experimental studies demonstrated that mechanisms of the LVH regression after RDN are not limited to the decrease in sympathetic activity and blood pressure. The levels of expression of myocardial TNF, IL-6, TLR-4 may also be relevant in this regard. An experimental study of RDN performed in spontaneously hypertensive rats has shown that compared to the control Wistar Kyoto spontaneously hypertensive rats had markedly higher blood pressure, LVMI and protein expression of TLR4, NF-κB, TNF-α and IL-6 in the myocardium, which were significantly reduced after RDN in contrast with sham-operated animals [14].
In 2015 a group of German authors published a study that documented a significant decrease in IL-6 and CRP whereas an increase in matrix metalloproteinases (MMP-2, MMP-9) at 6 months after RDN [15].
The main finding of our study is that the IVS thickness and LVM assessed by echocardiography decreased significantly at 24 months after RDN. Thus, the RDN in RH patients may have long-term cardioprotective effect, which at least in part may be attributed to the regress in systemic or myocardial inf lammatory activity. In spite of the research limitations (small sample, absence of control group) the results obtained are scientific interest and demand continuing the research.
References
1. Bramlage P, Hasford J. Blood pressure reduction, persistence and costs in the evaluation of antihypertensive drug treatment — a review. Cardiovasc Diabetol. 2009;27:8-18. doi:10.1186/1475-2840-8-18.
2. Cuspidi C, Vaccarella A, Negri F, et al. Resistant hypertension and left ventricular hypertrophy: an overview. J Am Soc Hypertens. 2010;4(6):319-4. doi:10.1016/j.jash.2010.10.003.
3. Kannel WB, Sorlie P, Castelli WP, et al. Blood pressure and survival after myocardial infarction: the Framingam study. Am J Cardiol. 1980;45(2):326-30. doi:10.1016/0002-9149(80)90654-2.
4. Koyanagi S, Eastham CL, Harrison DG, et al. Increased size of myocardial infarction in dogs with chronic hypertension and left ventricular hypertrophy. Circulation Research. 1982;50(1):55-62. doi:10.1161/01.res.50.1.55.
5. Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43(12):2207-15. doi:10.1016/j.jacc.2003.11.064.
6. Gerdts E, Wachtell K, Omvik P, et al. Left atrial size and risk of major cardiovascular events during antihypertensive treatment Losartan intervention for endpoint reduction in hypertension trial. Hypertension. 2007;49(2):311-6. doi: 10.1161/01.hyp.0000254322.96189.85.
7. Lu D, Wang K, Liu Q, et al. Reductions of left ventricular mass and atrial size following renal denervation: a meta-analysis. Clin Res Cardiol. 2016;105(8):648-56. doi:10.1007/s00392-016-0964-2.
8. Sitkova ES, Mordovin VF, Ripp TM, et al. Positive effects of renal denervation on left ventricular hypertrophy and subendocardial damage. “Arterial’naya Gipertenziya” (“Arterial Hypertension”). 2019;25(1):46-59. (In Russ.) doi:10.18705/1607-419X-2019-25-1-46-59.
9. Tsioufis C, Papademetriou V, Dimitriadis, et al. Long-term effects of multielectrode renal denervation on cardiac adaptations in resistant hypertensive patients with left ventricular hypertrophy. J Hum Hypertens. 2016;30(11):714-9. doi:10.1038/jhh.2015.127
10. Dinh QN, Drummond GR, Sobey CG, et al. Roles of inflammation, oxidative stress and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960. doi:10.1155/2014/406960.
11. Bautista LE, Vera LM, Arenas IA, et al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Human Hypertens. 2005;19(2):149-54. doi:10.1038/sj.jhh.1001785.
12. Mehta SK, Rame JE, Khera A, et al. Left Ventricular Hypertrophy, Subclinical Atherosclerosis, and Inflammation. Hypertens 2007;49(6):1385-91. doi:10.1161/hypertensionaha.107.087890.
13. Blake GJ, Rifai N, Buring JE, et al. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003; 108(24):2993-9. doi:10.1161/01.cir.0000104566.10178.af.
14. Jiang W, Tan L, Guo Y, et al. Effect of renal denervation procedure on left ventricular hypertrophy of hypertensive rats and its mechanisms. Acta Cir Bras. 2012;27(11):815-20. doi:10.1590/S0102-86502012001100012.
15. Dorr O, Liebetrau C, Mollmann H, et al. Beneficial effects of renal sympathetic denervation on cardiovascular inflammation and remodeling in essential hypertension. Clin Res Cardiol. 2015;104(2):175-84. doi:10.1007/s00392-014-0773-4.
About the Authors
E. S. SitkovaРоссия
PhD, researcher, department of hypertension.
Tomsk
V. F. Mordovin
Россия
Professor, PhD, the head of department of hypertension.
Tomsk
S. E. Pekarskiy
Россия
PhD, Senior research fellow, department of hypertension.
Tomsk
T. M. Ripp
Россия
PhD, Senior research fellow, department of hypertension.
Tomsk
T. R. Ryabova
Россия
PhD, researcher, department ultrasound and functional diagnostic.
Tomsk
А. Yu. Falkovskaya
Россия
PhD, Senior research fellow, department of hypertension.
Tomsk
V. A. Lichikaki
Россия
PhD, researcher, department of hypertension.
Tomsk
I. V. Zyubanova
Россия
PhD, junior researcher, department of hypertension.
Tomsk
A. Е. Baev
Россия
PhD, head of the Department of Interventional Radiology.
Tomsk
A. M. Gusakova
Россия
PhD, researcher, Department of Laboratory and Functional Diagnostics.
Tomsk
Supplementary files
Review
For citations:
Sitkova E.S., Mordovin V.F., Pekarskiy S.E., Ripp T.M., Ryabova T.R., Falkovskaya А.Yu., Lichikaki V.A., Zyubanova I.V., Baev A.Е., Gusakova A.M. Positive effects of renal denervation on markers of cardiovascular inflammation and left ventricular mass. 24-months follow-up. Cardiovascular Therapy and Prevention. 2021;20(2):2678. https://doi.org/10.15829/1728-8800-2021-2678
JATS XML

























































