Scroll to:
White matter integrity of watershed areas is potentially influenced by hypoperfusion in the presence permanent atrial fibrillation
https://doi.org/10.15829/1728-8800-2021-2915
Abstract
Aim. To test a hypothesis of hypoperfusion-induced white matter changes in patients with atrial fibrillation (AFib) and to present statistics to compute sample size for the upcoming studies.
Material and methods. We included 30 inpatients with AFib and investigated them with magnetic resonance imaging (MRI) with standard sequencies and diffusion tensor imaging (DTI). DTI data were analyzed with conventional ROI analysis in the Olea Sphere software and with watershed areas (WSA) mask in the FSL toolbox after nonlinear transformation of images to the Montreal Neurological Institute (MNI) space. Wilcoxon test was used to compare diffusion characteristics across subgroups.
Results. Median age of participants was 73 years (69-78), 18 (60%) patients had moderate signs of small vessel disease with Fazekas score of one. Twenty-one patients had paroxysmal AFib. Analysis of WSA revealed decreased white matter integrity in the parieto-occipital cortical WSA with a pattern of significantly increased mean diffusivity (p=0,039), and marginally significant decrease in fractional anisotropy (p=0,056). Rank-based effect size across areas under comparison was either small (0,2) or negligible, and with statistical power in the range of 0,05-1.
Conclusion. Atrial fibrillation could have pathophysiologically feasible mechanism to affect white matter integrity in the watershed areas.
Keywords
For citations:
Krupenin P.M., Perepelov V.A., Perepelova E.M., Bordovsky S.P., Sidorov E.V., Preobrazhenskaya I.S., Voskresenskaya O.N., Sokolova A.A., Napalkov D.A. White matter integrity of watershed areas is potentially influenced by hypoperfusion in the presence permanent atrial fibrillation. Cardiovascular Therapy and Prevention. 2021;20(4):2915. https://doi.org/10.15829/1728-8800-2021-2915
Introduction
Atrial fibrillation (AFib) imposes the greatest socioeconomic burden among other types of arrhythmias [1]. It significantly contributes to the risk of cerebral and myocardial infarction and decreases quality of life. AFib may result in cardioembolic stroke but may also alter brain perfusion and lead to small vessel disease (SVD).
There are certain brain regions that are the most susceptible to ischemia due to unique properties of their arterial supply. These zones are located between the distal parts of two arteries which have no anastomosis. The lower perfusion pressure in this region defines the higher susceptibility to ischemia. Moreover, the presence of microemboli and their derivates within the watershed areas in the autopsy series proves the association between the embolic events and development of infarction in vulnerable zones [2].
Hypoperfusion of these areas may be potentially accompanied by embolism which leads to impaired clearance of the substrate [3]. Both of these mechanisms could be seen in patients with AFib [4].
Two different types of watershed zones could be defined. The first one is a cortical watershed area (WSA) located at the junction of major brain arteries zones — between anterior cerebral artery (ACA) and middle cerebral artery (MCA) and between MCA and posterior cerebral artery (PCA). Acute lesions in this area are usually visualised by magnetic resonance imaging (MRI) as wedge-shaped or ovoid. The principal ischemia mechanism in the cortical WSA is an embolic event due to hemodynamic factors are less likely to cause alterations in these areas [5].
The second type of watershed area is internal WSA primarily located in the region of lenticulostriate vessels, cortical and deep branches of the MCA. This area was shown to be susceptible to hemodynamic alterations more likely than to embolism. Blood supply of subcortical white matter can explain high susceptibility of these zones to ischemia. Firstly, medullary penetrating vessels (from middle and anterior cerebral arteries) which supply these zones are the most distal branches of the internal carotid arteries and hence have the lowest perfusion pressure. Secondly, the deep perforating lenticulostriate arteries (which also supply these zones) have little collateral blood flow. Thirdly, there are no anastomoses between both vessel types. Consequently, corona radiata and centrum semiovale are the most vulnerable brain zones to hemodynamic damage [5].
Currently there is no definite opinion on AFibassociated impact on brain white matter because studies have to address high heterogeneity of patient population and variety of white matter changes. The study of community dwelling individuals showed no association of AFib with brain diffusion characteristics [6]. Other studies have demonstrated negative association of brain health and AFib [7], but also revealed stronger impact of permanent AFib [8]. However, there are no studies of white matter diffusion properties inside watershed areas of the brain. There is also a lack of data for calculating sample size of costeffective studies with diffusion tensor imaging (DTI) implementation. Hence, we conducted a pilot study in order to investigate association of AFib and white matter change in watershed areas.
Material and methods
Patients. We evaluated patients admitted to Sechenov Hospital #1 for management of Afib. All patients provided written informed consent and the study was approved by the local ethic review board. Inclusion criteria were as follows: 1) Age ≥65 years, 2) AFib treated with direct oral anticoagulants (DOACs). Non-inclusion criteria were conditions that potentially affects white matter integrity: 1) stroke in the past, 2) poorly controlled diabetes mellitus and/or hypertension, 3) lifetime smoking history of >100 cigarettes, 4) body mass index >30. All patients underwent screening ultrasound of head and neck vessels to detect significant atherosclerosis. If AFib lasted for ≥6 months it was considered to be permanent.
MRI. All subjects underwent brain MRI on a 3.0T MAGNETOM Skyra MR-scanner (Siemens AG, Germany) with standardized MRI protocol. In addition to standard pulse sequences, it included diffusion tensor imaging with following properties: DTI SE EPI (spin-echo echo-planar imaging) MDDW (Multi-Directional Diffusion Weighting). Diffusion gradients were applied along 30 noncollinear directions with a b value of 0 s/mm2 and 1000 s/mm2, symmetric FoV 220 mm, matrix 128×128 pixels, slice thickness 4 mm, TR 3700 ms, TE 92 ms, NEX 1; MRI data was assessed independently by two neuroradiologists in regard to markers of small vessel disease and Fazekas grading.
MRI processing. DTI data were processed in Mrtrix3 [9] and The FMRIB Software Library (FSL) toolbox as described previously [10]. In brief, images were denoised and motion corrected, then the non-brain tissues were stripped in FSL Brain Extraction Tool [11], and finally diffusion tensors were fitted in FSL FMRIB’s Diffusion Toolbox [12]. Individual DTI images were nonlinearly registered to Montreal Neurological Institute (MNI) standard space. For proper segmentation of watershed zones an empirical approach was implemented with the guidance of MNI vascular territories mask [13]. Right hemisphere external and internal watershed zones were manually segmented in ITK-SNAP and then they were mirrored to the contralateral hemisphere (Figure 1). Later this mask was used as a template to detect fractional anisotropy values in the following regions of interest: external watershed cortical areas (anterior cerebral and middle cerebral arteries; posterior and middle cerebral arteries) and internal watershed areas (mainly lenticulostriate and middle cerebral artery).
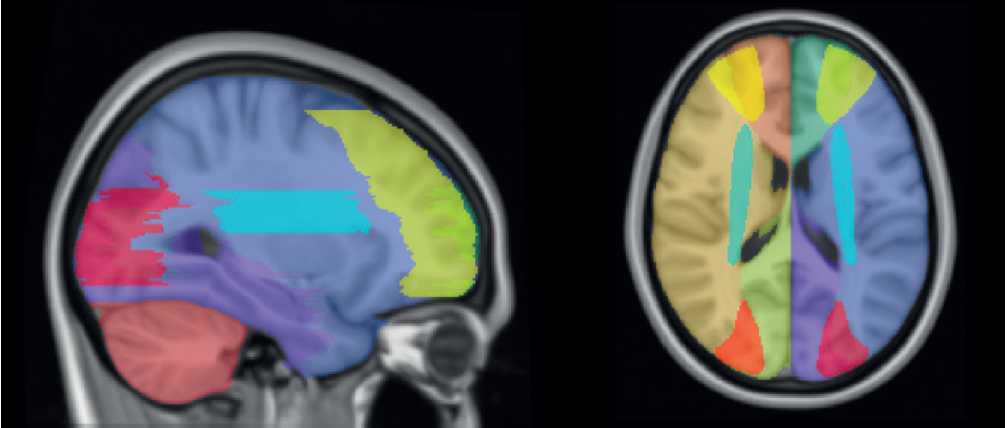
Figure 1. Segmentation of watershed areas according to vascular territories.
Manual ROI analyses. Manual ROI analysis was carried out with Olea Sphere 3.0-SP23 DTI plug-in, a part of MRI post-processing package by Olea Medical (Figure 2). Anterior thalamic radiation (ATR), corticospinal tract (CST), and inferior longitudinal fasciculus (ILF) were chosen to represent ACA-MCA, internal, and MCA-PCA watershed zones, respectively. ROI seeds were placed by trained neurologist according to the DTI tract atlas [14]. Initially, a larger seed was placed in the area of interest on a coregistered anatomical T1 image to reconstruct all of the fibers passing through the seed both on the anatomical image and on the diffusion map. Then, a more precise seed was placed in the ROI according to atlas data, reconstructed fibers, and anatomy.
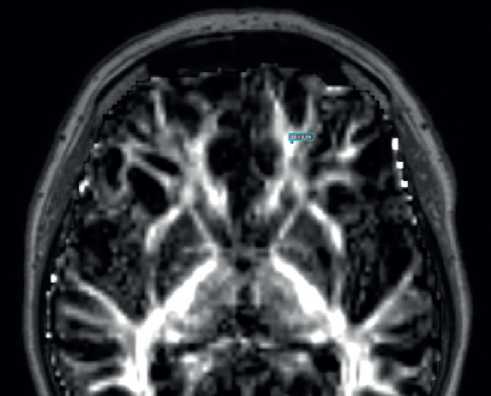
Figure 2. An example of manual ROI analysis. A seed of 0,1 cm2 is placed in the anterior thalamic radiation which corresponds to frontal watershed area.
Statistical analyses. Data were analysed in R (version 4.0.3 under RStudio Version 1.3.1093). Non-parametric Wilcoxon Rank test was implemented to compare subgroup of patients in regard to diffusion values inside ROIs. P values <0,05 were considered to be significant. Statistical power was calculated with help of effectsize [15] R package to calculate rank based effect size and wmwpow [16] package was used to calculate power.
Results
Thirty patients underwent the evaluation, the median age was 73 (IQR 69-78) years, 21 individuals had paroxysmal AFib, Table 1. None of the patients had major cognitive impairment as all individuals were independent in the activities of daily living. Most of the patients 18 (60%) had deep white matter and periventricular hyperintensities, which were rated at one point on a Fazekas scale. Analysis of manually drawn ROI in ATR, CST, and ILF did not reveal differences between patients with paroxysmal and permanent AFib (data not shown). Analyses according to watershed areas in MNI space found a significant difference between permanent and paroxysmal AFib subgroups in the region of the left parieto-occipital cortical watershed zone (Table 2). The pattern of marginally different FA (p=0,056) and significantly different MD (p=0,039) was revealed. Other areas either had equal diffusion tensor characteristics, or no conclusion regarding H0 could be drawn (Table 2). Diffusion values in the current sample had modest variability with subsequent low or negligible effect size.
Table 1
Demographic and clinical characteristics of the group
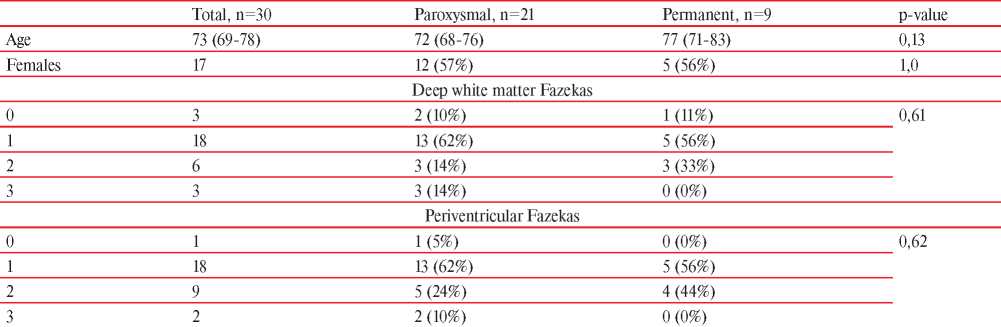
Note: continuous variables are presented as median with interquartile range.
Table 2
Diffusion characteristics across WSA with corresponding effect size and power
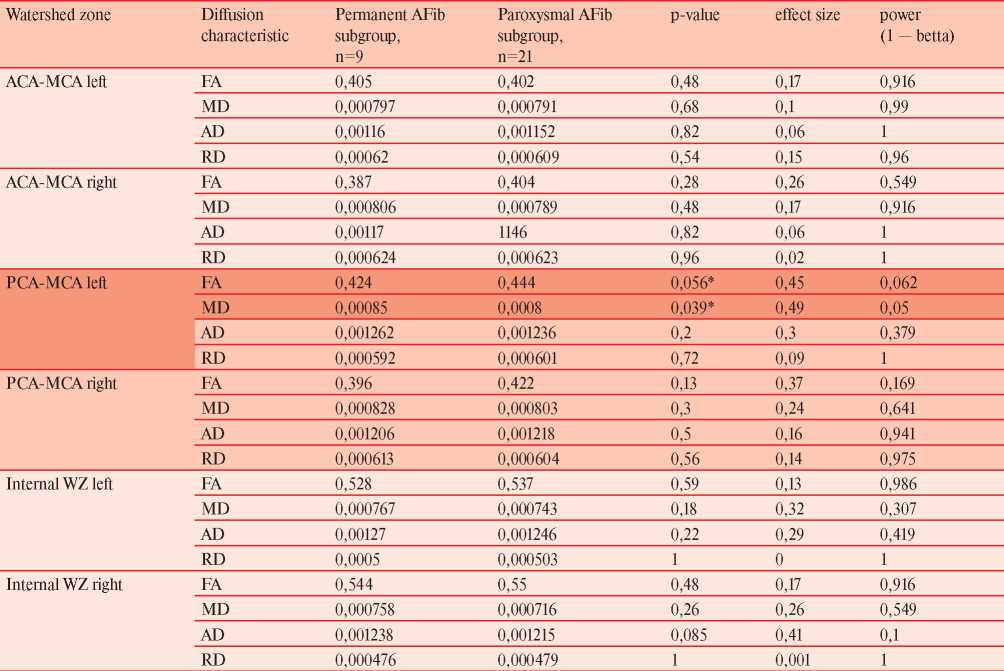
Note: * — p-values with borderline significant (0,056) and significant (0,039) values; ACA — anterior cerebral artery, AD — axial diffusivity, FA — fractional anisotropy, MCA — middle cerebral artery, MD — mean diffusivity, PCA — posterior cerebral artery, RD — radial diffusivity, WSA — watershed area.
Discussion
Atrial fibrillation broadly contributes to socalled “brain health” and not only increases risk of stroke but potentially could be involved in cerebral small vessel disease via several mechanisms. Interaction of altered cardiac cycle and properties of cerebral blood supply is a pathologically plausible mechanism of SVD, and the additive effect of microembolism further increases the risks of brain damage. Our data suggest a decreased white matter integrity in one of the cortical WSA at the MCAPCA junction in patients with permanent AFib, but no cause for this lateralization could be drawn from the data. The pattern of increased MD and decreased FA was previously shown to be associated with altered composition of white matter and its demyelination [17].
According to the individual blood supply features, the WSA location can have interindividual variations [18]. This feature of cerebral vasculature requires MR data to be normalized to increase validity of the analysis being performed and strengthen the statistical power of such analysis in small sample studies.
Current study was implemented to test the hypothesis of altered white matter integrity in WSA in patients with permanent AFib due to pathophysiologic mechanism of hypoperfusion. Another aim of this study was to evaluate a feasibility of that kind of analysis in a relatively small subset of patients. Our data has shown that values of diffusion characteristics do not have broad variability in a selected group of individuals — a feature that should be taken into consideration in upcoming studies. Current data suggest that preliminary costeffective sample of 30 individuals allows for hypothesis testing with help of nonparametric tests. Also, current study did not correct for multiple comparisons, the amount of calculation carried out is small and was performed in previously defined areas.
Conclusion
Atrial fibrillation could contribute to cerebral microstructure integrity via multiple mechanisms, and interaction of altered cardiac cycle and inherent properties of cerebral circulation is a plausible pathogenetic mechanism for small vessel disease manifestation in watershed areas. Our data suggest an altered white matter integrity in one of the cortical watershed areas in individuals with permanent fibrillation, but no causality could be implied. This concept should be addressed in future studies with larger sample size. It is possible to perform analysis of DTI data in small sample, cost-effective studies to draw conclusions with statistical power of ≥80% or to decline the H0 hypothesis with rank-based nonparametric tests even though the effect size is either small or negligible.
References
1. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke [Internet]. 2021 Feb 19;16(2):217–21. Available from: 10.1177/1747493019897870
2. Masuda J, Yutani C, Ogata J, Kuriyama Y, Yamaguchi T. Atheromatous embolism in the brain. Neurology [Internet]. 1994 Jul;44(7):1231–1231. Available from: 10.1212/WNL.44.7.1231
3. Caplan LR, Hennerici M. Impaired Clearance of Emboli (Washout) Is an Important Link Between Hypoperfusion, Embolism, and Ischemic Stroke. Arch Neurol [Internet]. 1998 Nov 1;55(11):1475. Available from: 10.1001/archneur.55.11.1475
4. Gardarsdottir M, Sigurdsson S, Aspelund T, Rokita H, Launer LJ, Gudnason V, et al. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. EP Eur [Internet]. 2017;1–7. Available from: 10.1093/europace/eux220
5. Mangla R, Kolar B, Almast J, Ekholm SE. Border Zone Infarcts: Pathophysiologic and Imaging Characteristics. RadioGraphics [Internet]. 2011 Sep;31(5):1201–14. Available from: 10.1148/rg.315105014
6. Shao IY, Power MC, Mosley T, Jack C, Gottesman RF, Chen LY, et al. Association of Atrial Fibrillation With White Matter Disease. Stroke [Internet]. 2019 Apr;50(4):989–91. Available from: 10.1161/STROKEAHA.118.023386
7. Mayasi Y, Helenius J, McManus DD, Goddeau RP, Jun-O’Connell AH, Moonis M, et al. Atrial fibrillation is associated with anterior predominant white matter lesions in patients presenting with embolic stroke. J Neurol Neurosurg Psychiatry [Internet]. 2018 Jan;89(1):6–13. Available from: 10.1136/jnnp-2016-315457
8. Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, et al. Atrial Fibrillation is Associated With Reduced Brain Volume and Cognitive Function Independent of Cerebral Infarcts. Stroke [Internet]. 2013 Apr;44(4):1020–5. Available from: 10.1161/STROKEAHA.12.679381
9. Tournier J-D, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage [Internet]. 2019 Nov;202:116137. Available from: 10.1016/j.neuroimage.2019.116137
10. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage [Internet]. 2006 Jul;31(4):1487–505. Available from: 10.1016/j.neuroimage.2006.02.024
11. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp [Internet]. 2002 Nov;17(3):143–55. Available from: 10.1002/hbm.10062
12. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. Neuroimage [Internet]. 2012;62(2):782–90. Available from: 10.1016/j.neuroimage.2011.09.015
13. Kenichi Oishi, Andreia V. Faria, Peter C M van Zijl SM. MRI Atlas of Human White Matter-Academic Press [Internet]. Elsevier B.V.; 2011. 266 p, ISBN 9780123820822.
14. Ben-Shachar M, Lüdecke D, Makowski D. effectsize: Estimation of Effect Size Indices and Standardized Parameters. J Open Source Softw [Internet]. 2020 Dec 23;5(56):2815. Available from: 10.21105/joss.02815
15. Mollan KR, Trumble IM, Reifeis SA, Ferrer O, Bay CP, Baldoni PL, et al. Precise and accurate power of the rank-sum test for a continuous outcome. J Biopharm Stat [Internet]. 2020 Jul 3;30(4):639–48. Available from: 10.1080/10543406.2020.1730866
16. Pasi M, van Uden IWM, Tuladhar AM, de Leeuw F-E, Pantoni L. White Matter Microstructural Damage on Diffusion Tensor Imaging in Cerebral Small Vessel Disease. Stroke [Internet]. 2016 Jun;47(6):1679–84. Available from: 10.1161/STROKEAHA.115.012065
17. van der Zwan A, Hillen B. Review of the variability of the territories of the major cerebral arteries. Stroke [Internet]. 1991 Aug;22(8):1078–84. Available from: 10.1161/01.STR.22.8.1078
About the Authors
P. M. KrupeninРоссия
Pavel Mikhaylovich Krupenin — MD
V. A. Perepelov
Россия
Vsevolod Andreevich Perepelov— MD
E. M. Perepelova
Россия
Elena Mikhaylovna Perepelova — MD, PhD
S. P. Bordovsky
Россия
Sergey Petrovich Bordovsky — MD
E. V. Sidorov
Соединённые Штаты Америки
Evgeny Vadimovich Sidorov — MD, PhD, Professor.
Oklahoma City
I. S. Preobrazhenskaya
Россия
Irina Sergeevna Preobrazhenskaya — MD, PhD, Professor
O. N. Voskresenskaya
Россия
Olga Nikolaevna Voskresenskaya — MD, PhD, Professor
A. A. Sokolova
Россия
Anastasiya Andreevna Sokolova — MD, PhD, Professor
D. A. Napalkov
Россия
Dmitry Alexandrovich Napalkov — MD, PhD, Professor
Review
For citations:
Krupenin P.M., Perepelov V.A., Perepelova E.M., Bordovsky S.P., Sidorov E.V., Preobrazhenskaya I.S., Voskresenskaya O.N., Sokolova A.A., Napalkov D.A. White matter integrity of watershed areas is potentially influenced by hypoperfusion in the presence permanent atrial fibrillation. Cardiovascular Therapy and Prevention. 2021;20(4):2915. https://doi.org/10.15829/1728-8800-2021-2915
JATS XML

























































