Scroll to:
Long-term dynamics of the levels of anti-SARS-CoV-2 S-protein IgG antibodies in vaccinated individuals
https://doi.org/10.15829/1728-8800-2021-3124
Abstract
In the context of the ongoing coronavirus disease 2019 (COVID-19) pandemic, it is extremely important to study immunogenicity and immune response duration in different vaccines.
Aim. As part of a prospective observational study, to study the levels of anti-SARS-CoV-2 S-protein IgG antibodies in individuals vaccinated with the Gam-COVID-Vac and CoviVac vaccines.
Material and methods. The data of 93 people who completed the first 3 visits were analyzed, 23 of whom were vaccinated with the Gam-COVID-Vac vaccine and 70 people — with the CoviVac vaccine. We collected blood before the injection of vaccine doses I and II, as well as 42 days after the injection of dose I in order to quantitatively determine IgG levels. The level of anti-SARS-CoV-2 S-protein IgG antibodies was determined using the SARS-CoV-2 IgG ELISA-BEST reagent kit on the InfiniteF50 TECAN system.
Results. A significant increase in anti-SARS-CoV-2 S-protein IgG antibodies was observed in those vaccinated with Gam-COVID-Vac. In the group of CoviVac vaccine, an increase in the level anti-SARS-CoV-2 S-protein IgG antibodies in absolute values was recorded, however, this increase did not reach statistical significance.
Conclusion. The data obtained show that the level of anti-SARS-CoV-2 S-protein antibodies 42 days after Gam-COVID-Vac vaccination is significantly higher than after CoviVac vaccination. However, an increase in the level of IgG in both groups indicates the ability of both vaccines to stimulate the production of anti-SARS-CoV antibodies.
Keywords
For citations:
Drapkina O.M., Burns S.A., Gorshkov A.Yu., Shishkova V.N., Ryzhakova L.N., Litinskaya O.A., Ivanova A.A., Veretennikova A.V., Bashnyak V.S., Tatarevich E.Yu. Long-term dynamics of the levels of anti-SARS-CoV-2 S-protein IgG antibodies in vaccinated individuals. Cardiovascular Therapy and Prevention. 2021;20(8):3124. https://doi.org/10.15829/1728-8800-2021-3124
Introduction
At the end of 2019, an outbreak of сoronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in the People’s Republic of China [1]. Due to the extremely rapid increase in the number of infected people around the world, in the spring of 2020, the disease was recognized as a pandemic.
According to official statistics, as of November 2021, more than 246 million COVID-19 cases were registered in the world, of which 4,99 million were fatal [2]. At the time of writing (November 30, 2021), more than 9,64 million people were infected in Russia, of which more than 275 thousand people died [3]. To stop the further growth of pandemic and reduce health system loads, the development of specific prevention of COVID-19 has become a priority for scientists around the world. In the context of COVID-19 pandemic, when the number of cases worldwide, including in Russia, continues to grow, one of the highly effective prevention is mass vaccination of the population. Currently, the following vaccines have been registered in Russia for the specific COVID-19 prevention in adults: GamCOVID-Vac, combined vector vaccine; Gam-COVIDVac-Lio, combined vector vaccine; EpiVacCorona, peptide-based vaccine; CoviVac, inactivated wholevirion concentrated, purified coronavirus vaccine; Sputnik Light, the vaccine for COVID-19 prevention; EpiVacCorona-N, peptide-based vaccine [4].
One of the world’s first vaccines against COVID-19 was the Russian vaccine Gam-COVID-Vac, developed at the Gamaleya Research Institute of Epidemiology and Microbiology. Gam-COVID-Vac is a biotechnological vector vaccine that does not use the SARS-CoV-2 pathogenic for humans. The vaccine consists of two doses: dose I and dose II. Dose I includes a recombinant adenovirus type 26, carrying the SARS-CoV-2 S protein gene, while dose II includes a human adenovirus type 5, also carrying the SARS-CoV-2 S protein gene [1]. Both doses are administered intramuscularly, separately, with an interval of 21 days.
It is known that the use of adenoviral vectors for the rapid delivery of antigens induces both cellmediated and humoral immunity even after a single injection, while the use of two vaccine doses makes it possible to obtain a reliable and long-term immune response [5]. Phases I-II of Gam-COVID-Vac clinical trials, completed by the beginning of August 2020, showed high immunogenicity and, importantly, good tolerability in healthy participants. It is important to note that the number of side effects did not differ from those for other similar vector vaccines, while no serious adverse events were observed [6]. Phase III results have been published in The Lancet journal. The use of GamCOVID-Vac showed an effectiveness of 91,6% (95% confidence interval, 85,6-95,2) and a high safety profile. Anti-SARS-CoV-2 neutralizing IgG antibodies after Gam-COVID-Vac vaccination were found in 98% of volunteers [7].
Efficacy and safety of vaccine were also confirmed in real-world evidence studies [8]. None of the 204 vaccinated with Gam-COVID-Vac developed serious adverse events within 42 days after dose I introduction, and IgG positive rate was 16,2.
Another CoviVac vaccine approved in Russia is a purified concentrated SARS-CoV-2 (AYDAR-1 strain) suspension. The CoviVac vaccine has shown high efficacy and safety in preclinical studies in rodents and primates [9], and phase III clinical trials are currently being completed.
Given the variety of existing vaccines, it is currently extremely important to study their immunogenicity and the duration of immune response.
The aim of the study is to assess the immune status in individuals vaccinated against the SARS-CoV-2 as part of a prospective observational study.
It is planned to evaluate the levels of anti-SARSCoV-2 S-protein antibodies in vaccinated individuals before dose 1 introduction, before dose 2 introduction, and also 42 days, 3, 6 and 12 months after the dose 1 introduction. The study collects and analyzes data on the tolerability of anti-SARS-CoV-2 vaccines, as well as clinical information on study participants (anthropometric parameters, social status, physical examination, comorbidities, medications taken, etc.). In the present work, the data of those patients who completed the first 3 visits (day 0, day 14-21, day 42nd) are analyzed.
Material and methods
This paper presents the first results of the ongoing SIRIUS study.
Inclusion criteria:
- age ≥18 years;
- no contraindications to vaccination;
- residents of Moscow and Moscow region;
- signed informed consent.
Exclusion criteria:
- contraindications to vaccination;
- prior COVID-19 infection;
- prior anti-SARS-CoV-2 vaccination;
- refusal to participate in the study.
As of November 1, 2021, 1090 people were included in the SIRIUS study, of which 93 people completed the first 3 visits: 23 people were vaccinated with the Gam-COVIDVac, 70 people — CoviVac. Patients had the opportunity to choose the vaccine. Venous blood samples were taken before the introduction of dose I and II, as well as 42 days after the dose I introduction in order to quantify the level of S protein IgG antibodies. During the time elapsed between visits, study participants had no contact with COVID-19 patients.
Protocol № 05-07/21 dated June 3, 2021 was approved by the local ethics committee of the National Medical Research Center for Therapy and Preventive Medicine, and complies with the Declaration of Helsinki principles. Signed informed consent was obtained from all patients.
The level of serum immunoglobulins was determined using the SARS-CoV-2 IgG ELISA-BEST kit to assess the humoral immune response to current or past SARS-CoV-2 infection, as well as to assess vaccine-induced immunity based on the S-protein (including the receptor domain binding (RDB)) of SARS-CoV-2. For this purpose, the InfiniteF50 TECAN system was used. When quantifying anti-SARS-CoV-2 S protein IgG antibodies, the reference value of 10 binding antibody units (BAU)/ml was used. Values <10 BAU/ml are negative (no antibodies or below the limit of detection), while values >10 BAU/mL — positive.
For statistical processing, the program R, SPSS v.20.0 was used. The differences were considered significant at p<0,05. The normality of distribution was assessed using the Shapiro-Wilk and Kolmogorov-Smirnov tests with the Lilliefors correction. Variables were presented as Me (Q25; Q75), where Me is the median, Q25 — 25th percentile, Q75 — 75th percentile. For proportions and frequencies, data are presented as a percentage. Nonparametric criteria were used to compare groups as follows: independent groups — MannWhitney test, dependent groups — Wilcoxon test.
Results
The study included 93 patients, of whom 23 were vaccinated with Gam-COVID-Vac and 70 with CoviVac. In the Gam-COVID-Vac group (group 1), the mean age of participants was 43 years (31; 58), in the CoviVac group (group 2) — 45 years (23; 53). In the 1st group, there were 30,4% of men, in the 2nd — 22,9%. Thus, the age and sex composition of groups did not differ. Participants in both vaccinated groups were also comparable in terms of body weight, height, body mass index, smoking status, and number of those vaccinated against influenza (Table 1).
Table 1
Characteristics of study participants
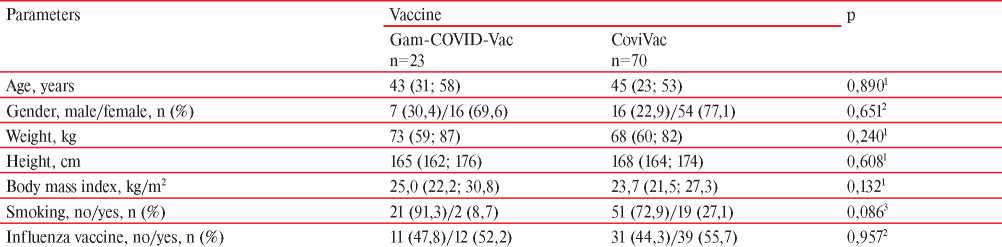
Note: data are presented as Me (Q25; Q75), where Me is the median, Q25 and Q75 — 25th and 75th percentiles, p — significance level, 1 — MannWhitney test, 2 — χ2 test with continuity correction, 3 — Fisher’s exact test.
An analysis was made on the level of S protein IgG antibodies in vaccinated individuals before the introduction of vaccine, before the dose 2 and on the 42nd day after dose 1 administration (Figure 1).
In the group 1, the Me level of S protein IgG antibodies at the first visit was 6 BAU/ml (Q25 — 5 BAU/ml; Q75 — 58 BAU/ml), in the group 2 — 7 BAU/ml ml (Q25 — 5 BAU/ml; Q75 — 63 BAU/ml), while there was no significant difference in the baseline level of S protein IgG antibodies between the studied groups (p=0,847). A reference level >10 BAU/mL is considered positive. At the second visit, there were significant differences in S protein IgG antibodies between the 1st and 2nd groups. In absolute values, the Me level of S protein IgG antibodies in group 1 was 250 BAU/ml (Q25 — 99 BAU/ml; Q75 — 540 BAU/ ml), while in group 2 — 7 BAU/ml (Q25 — 5 BAU/ml; Q75 — 162 BAU/ml). At the third visit, the Me level of S protein IgG antibodies in group 1 reached 527 BAU/ml (Q25 — 196 BAU/ml; Q75 — 580 BAU/ml), while in group 2 — 21 BAU/ml (Q25 — 7 BAU/ml Q75 — 210 BAU/ml). Differences between the two groups at the second and third visits were significant (p<0,001).
The results of the analysis of S protein IgG antibodies dynamics in both studied groups of vaccinated individuals are shown in Figure 2.
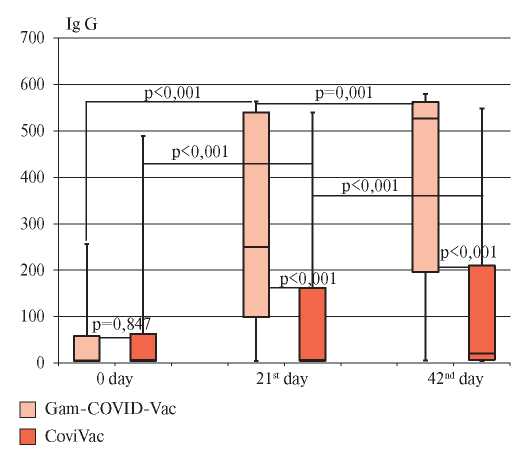
Figure 1. The level of IgG antibodies in the studied groups of vaccinated people during the follow-up period.
Note: “whiskers” — minimum and maximum values, “box” borders — Q25-Q75, line inside the “box” — median.
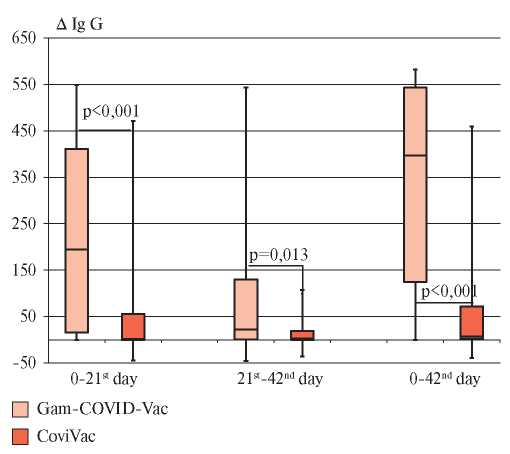
Figure 2. Dynamics of anti-S protein IgG antibodies in the studied groups of vaccinated people.
Note: “whiskers” — minimum and maximum values, “box” borders — Q25-Q75, line inside the “box” — median.
These data demonstrate that in group 1, compared with group 2, there was a pronounced and significant increase in the Me level S protein IgG antibodies, both when comparing the first and second visit, and when comparing the first and third visits (p<0,001). When comparing the differences in the levels of S protein IgG antibodies for the period from second to third visit, no significant increase was found for either Gam-COVID-Vac or CoviVac. Median increase in the level of S protein IgG antibodies 42 days after the Gam-COVID-Vac introduction was 397 BAU/ml.
Discussion
Thus, a pronounced and significant increase in S protein IgG antibodies was observed only in the 1st group. Despite the fact that in the 2nd group an increase in S protein IgG antibodies was recorded in absolute terms, this turned out to be statistically insignificant. It is likely that as the sample size increases, the results obtained will be reproducible.
Recently, novel data has appeared in The Lancet, confirming the safety, good tolerance and immunogenicity of the Sputnik Light vaccine. In an openlabel, prospective, non-randomized, phase I/II study, the main criteria for a positive vaccination result were antigen-specific humoral immunity (anti-RBDSARS-CoV-2 antibodies measured using ELISA on days 1, 10, 28, and 42nd) and safety (number of participants with adverse events). Secondary endpoints were antigen-specific cell mediated immunity (antigendependent proliferation of CD4+ and CD8+ T cells, number of antigen-specific cells producing interferon-γ, and interferon-γ concentration upon antigen restimulation) and change in neutralizing antibodies (measured using SARS-CoV-2 neutralization assay).
The study revealed mild adverse events in 66,4% of vaccinated people, moderate — in 5,5%, while no severe events were identified. Immunogenicity depended on the initial titer of antibodies. In 14 seropositive volunteers, RBD-specific IgG antibodies by the 10th day after vaccination increased from 594 to almost 27 thousand BAU/ml, while in 96 seronegative volunteers — to 29 thousand BAU/ml. By day 42nd, seroconversion, respectively, was 92,9%, while antigen-specific cell-mediated immunity developed in 100% of seropositive and 96% of seronegative volunteers [10].
After the CoviVac introduction, the vaccinated individuals showed an increase in the level of IgG antibodies in absolute terms, while the growth dynamics was not as pronounced as in the 1st group. The reason for this result may be the small sample size. More patients need to be analyzed and observed over a longer period. The authors emphasize that the results obtained do not establish the effectiveness of a particular vaccine. From the start of follow-up until the third visit, no new cases of COVID-19 infection were reported among the participants. Within the SIRIUS study, monitoring of patients observed in this paper is continued in order to assess the level of antibodies 3 months, six months and a year after the dose 1 introduction. The recruitment of patients in the SIRIUS study continues, which will soon allow repeating the analysis in a larger sample.
Conclusion
In the context of COVID-19 pandemic, it is important to continue studying the effects of vaccines against COVID-19, because vaccination is the only method of its specific prevention. The SIRIUS study is aimed at assessing the level of S protein antibodies in various periods after the vaccination. The data presented in this paper show that the level of anti-S protein antibodies 42 days after vaccination with Gam-COVID-Vac is significantly higher than after CoviVac vaccination. However, an increase in IgG levels was observed in both groups. In order to obtain more information about the immunogenicity of these vaccines, analysis of a larger number of patients is needed, as well as data on the level of anti-S protein antibodies at 3, 6, and 12 months after vaccination.
Relationships and Activities. The work was carried out within the State Assignment № 121093000069-9.
References
1. Livzan MA, Drapkina OM, Nikolaev NA, et al. Algorithms for adult outpatient care of coronavirus disease 2019 (COVID-19) and its Assumption. Cardiovascular Therapy and Prevention. 2021;20(4):2916. (In Russ.) doi:10.15829/1728-8800-2021-2916.
2. WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/.
3. Official dashboard: COVID-19 in Russia. (In Russ.) https://xn--80aesfpebagmfblc0a.xn--p1ai.
4. Avdeev SN, Adamyan LV, Alekseeva EI, et al. Prevention, diagnostics and treatment of coronavirus disease 2019 (COVID-19): Update 13 14.10.2021. Moscow, Ministry of Health of Russian Federation, 2021. 237 p. (In Russ.)
5. Dolzhikova IV, Zubkova OV, Tukhvatulin AI, et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother. 2017;13:613-20. doi: 10.9.1080/21645515.2016.1238535.
6. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and im-munogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887-97 doi:10.1016/S0140-6736(20)31866-3.
7. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al; Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671-81. doi:10.1016/S0140-6736(21)00234-8.
8. Drapkina OM, Ryzhakova LN, Shishkova VN, et al. First real-world experience of Gam-COVID-Vac Sputnik V vaccine use. The Russian Journal of Prevention Medicine. 2021;24(10):53-60. (In Russ.) doi: 10.17116/profmed20212410153.
9. Kozlovskaya LI, Piniaeva AN, Ignatyev GM, et al. Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies. Emerg Microbes Infect. 2021; 10(1):1790-806. doi:10.1080/22221751.2021.1971569.
10. Tukhvatulin AI, Dolzhikova IV, Shcheblyakov DV, et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg Health Eur. 2021; 11:100241. doi:10.1016/j.lanepe.2021.100241.
About the Authors
O. M. DrapkinaRussian Federation
Moscow
S. A. Burns
Russian Federation
Moscow
A. Yu. Gorshkov
Russian Federation
Moscow
V. N. Shishkova
Russian Federation
Moscow
L. N. Ryzhakova
Russian Federation
Moscow
O. A. Litinskaya
Russian Federation
Moscow
A. A. Ivanova
Russian Federation
Moscow
A. V. Veretennikova
Russian Federation
Moscow
V. S. Bashnyak
Russian Federation
Moscow
E. Yu. Tatarevich
Russian Federation
Moscow
Supplementary files
Review
For citations:
Drapkina O.M., Burns S.A., Gorshkov A.Yu., Shishkova V.N., Ryzhakova L.N., Litinskaya O.A., Ivanova A.A., Veretennikova A.V., Bashnyak V.S., Tatarevich E.Yu. Long-term dynamics of the levels of anti-SARS-CoV-2 S-protein IgG antibodies in vaccinated individuals. Cardiovascular Therapy and Prevention. 2021;20(8):3124. https://doi.org/10.15829/1728-8800-2021-3124

























































